Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Latin American applied research
versión impresa ISSN 0327-0793
Lat. Am. appl. res. v.32 n.3 Bahía Blanca jul./sept. 2002
Analysis of slag foaming during the operation of an industrial converter
C. Cicutti†, M. Valdez†, T. Pérez†, R.Donayo‡, J. Petroni‡
† Centro de Investigación Industrial, FUDETEC, J. Simini 250 (2804) Campana, Argentina
sidcci@siderca.com
‡ Gerencia de Areas Primarias, SIDERAR, CC 801 (2900) San Nicolás, Argentina
Abstract — In the converter operation, a proper control of the slag-metal reactions that take place along the process is required to guarantee successful results. During the decarburization reaction a high gas flow rate is generated which increases the slag volume and can, eventually, promote its spill out of the converter. In this work, samples of slag and metal were taken out of an industrial converter at different stages of the process using a special device. The evolution of slag weight and composition was determined. Furthermore, calculations were performed in order to estimate the foaming capacity of the slags at the different stages of the process. It was found that the foam height reaches a maximum in the first half of the process, mainly due to the higher slag viscosity.
Keywords — Steelmaking, Converter, Slags, Foaming.
I. INTRODUCTION
Despite of the progress that Electric Arc Furnace technology has evidenced over the last years, Oxygen Steelmaking is still used to produce more than 50 % of the total crude steel all around the world (Faure, 1993). In this process, the oxygen blown is mostly combined with the elements dissolved in the melt. Some of these elements (like Mn, Si and P) are oxidised and incorporated into the slag (Turkdogan, 1996; Deo and Boom, 1993). In the case of carbon, the reaction promotes large amounts of CO and CO2 that have to be evacuated through the slag layer. If the gas bubbles remain in the slag for a long time its volume increases and can be partly spilt out of the converter. Therefore, a proper control of the slag-metal reactions that take place along the process is required to guarantee successful results.
Although the foaming capacity of the slags has been determined in different laboratory studies (Ito and Frue han, 1988a, b; Utigard and Zamalloa, 1993; Ghag et al., 1998; Wu et al., 1999), only a few attempts have been made to apply these results to industrial systems. Consequently, the aim of the present work was to estimate the foaming capacity of the slags during the operation of an industrial converter.
II. EXPERIMENTAL WORK
Trials were performed at Siderar steel plant (San Nicolás, Argentina) in a 200 ton industrial converter. It is an LD converter where inert gas is blown through the bottom (LBE system). A brief description of the process conditions employed during the trials and the different additions carried out along the blow are listed in Table 1.

Slag and metal samples were taken from the mouth of the converter at different times from the start of the blow. The sampling was carried out with the aid of a special device (van Horn et al., 1976) which enables the obtaining of slag and metal samples all at once, see Fig. 1. Only one sample was taken in each heat by interrupting the blow and dipping the sampler into the converter. Further details of the sampling procedure and the methods employed to analyse the metal and slag samples have been presented elsewhere (Cicutti et al., 1999; Cicutti et al., 2000).

III. RESULTS AND DISCUSION
A. Evolution of slag composition
Figure 2 shows the evolution of slag composition in a CaO´-SiO2´-FeO´ ternary system. The path followed by the slag during the process is similar to that observed by other researchers (van Horn et al., 1976; Kreijer and Boom, 1982). In the same figure, the isotherms reported in the literature for this system have also been plotted (Margot-Marette and Riboud, 1964; Mills and Keene, 1987).

Using the metal and slag silicon contents measured along the process, mass balance calculations were performed in order to estimate the evolution of slag weight (Cicutti et al., 1999), see Fig. 3. At the beginning of the process the slag weight is increased steeply, mainly due to the oxidation of the melt components (Fe, P, Si) and the additions of lime and dolomite. At this stage, the slag FeO content is relatively high, allowing part of the added lime to be dissolved into the slag. Near the middle of the process a drop of the FeO content is observed, which almost coincides with the period of maximum decarburization of the bath. The slag weight remains almost unchanged during this period. However, towards the end of the process, most of the carbon has been removed from the melt so the oxygen blown forms FeO which is incorporated into the slag. This promotes an increment of the slag FeO content and of the slag weight (see Fig. 2 and 3).

The lime and dolomite added during the process are not immediately incorporated into the slag. Figure 3 shows the evolution of free lime content during the process. Calculated lime dissolution rates are in good agreement with data previously published in the literature (Baptizmanskii, 1973).
B. Analysis of decarburization reaction
Figure 4 shows the evolution of carbon content along the process. The three different zones usually reported in the literature (Turkdogan, 1996; Deo and Boom, 1993) can be clearly distinguished along this curve. At the beginning of the process, most of the oxygen blown is combined with Si, P, Fe and Mn and, consequently, the decarburization rate is low. During the second period, the decarburization rate is almost constant and it is controlled by the oxygen supply. Calculations have shown (Cicutti et al., 2000) that nearly all the oxygen blown in this stage is consumed by the decarburization reaction. When the carbon content is reduced below a certain level, the decarburization process begins to be controlled by carbon diffusion (Turkdogan, 1996; Deo and Boom, 1993) and its rate decreases again.

C. Calculation of slag foaming
As mentioned before, the decarburization reaction generates large amounts of gas that increase the slag volume and can produce slopping. This capability of the slag to increase its volume is usually characterised by a ‘Foaming Index' (S), which represents the mean residence time of the gas in the slag layer and can be defined as:
| | (1) |
In this equation, h is the foam height, hO is the slag height before the foaming begins and vGS is the superficial gas velocity. Based on a dimensional analysis of their experimental data, Ito and Fruehan (1988a, b) developed an equation to estimate the foaming index of slags as a function of their physical properties
| | (2) |
where m is the viscosity, r is the density and s is the interface tension. Although physical properties of slags have been thoroughly studied (Slag Atlas, 1995), it is difficult to find data of industrial slags because they are multicomponent systems and their composition may vary from one plant to other. Consequently, in order to evaluate the foaming capacity in industrial systems it is necessary to estimate the physical properties of the slags using mathematical models. In this case, the density and the interface tension were calculated using the method proposed by Mills and Keene (1987):
|
| (3)
|
| | (4) |
In these equations, Xi, Vi, Mi and si are the molar fraction, molar volume, molecular weight and surface tension of each component. These data can be obtained from results published in the literature (Mills and Keene, 1987).
Viscosity of liquid slag was estimated using the model proposed by Riboud et al., (1981):
| | (5) |
where T is the temperature and the parameters A and B depend on the slag composition. It is important to note that slag viscosity can be strongly affected by the presence of second phases. Different expressions have been proposed in the literature to correct the viscosity by the presence of solid particles (Ito and Fruehan, 1988a, b; Turkdogan, 1983). In this paper, the following equation was adopted:
| | (6) |
where mO is the viscosity of the liquid slag and e is the volume fraction of second phases.
Calculations were performed in order to estimate the evolution of the Foaming Index during the blow. For these calculations it was considered that the temperature changes linearly along the process from 1350 to 1650 °C. This is in agreement with the results published by other researchers (Takawa et al., 1988; Traebert et al., 1999). Two different cases were analysed: a completely liquid slag and a slag with undissolved lime particles. In the last case, the results of free lime volume fraction measured in the samples (Fig. 3) were employed. Figure 5 shows the results of these calculations. The lower temperature at the beginning of the process promotes a higher slag viscosity, increasing the foaming capacity of the slag. Similarly, the presence of free lime particles raises the viscosity, giving a higher Foaming Index.

In order to estimate the foam height in the converter it is necessary to calculate the superficial gas velocity, see Eqn (1),. This velocity can be computed from the gas flow rate generated along the process. The total gas flow rate is mainly composed by the decarburization products (QG) and the inert gas blown through the bottom of the converter (QAr). Assuming that the pressure inside the converter is 1.5 atm, the superficial gas velocity can be calculated as follows:
| | (7) |
where SC is the converter section. The gas flow rate generated by the decarburization reaction can be estimated using the data of carbon content measured in the melt along the process (Fig. 4). Considering that the gas generated is mainly carbon monoxide, the following equation results:
| | (8) |
where WMet is the metal weight (192 tons) and dC/dt the rate of carbon concentration change. During the period of maximum decarburization the calculated superficial velocity is around 3.5 m/s, which is in good agreement with the value reported by Turkdogan (1996).
The calculated evolution of slag and foam height are shown in Fig. 6. The initial slag thickness (without foaming) was estimated using the results of slag weight (Fig. 3) and slag density, Eqn (3). The foam height was computed employing Eqn (1).
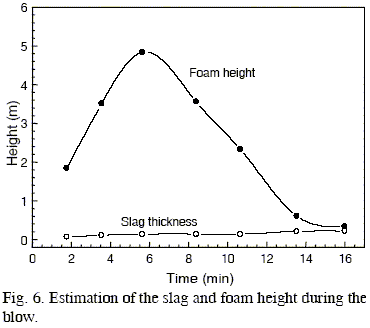
Due to the increase of slag weight along the process, a slight growth of the slag thickness is verified. On the other hand, the foam height exhibits a maximum in the first half of the blow. This is mainly caused by the high gas volume generated in the decarburization reaction and the high foaming capacity of the slag in this part of the process (Fig. 5). Consequently, conditions for slag slopping are more favourable during this period.
IV. CONCLUSIONS
Calculations were performed in order to estimate the foaming capacity of the slags at the different stages of the process. It was found that the slag Foaming Index is higher at the beginning of the process, mainly due to a higher slag viscosity. Both, the lower slag temperature and the presence of undissolved lime particles contribute to increase the slag viscosity in this part of the process. The gas flow rate generated by the decarburization reaction was calculated using the data of melt carbon content measured all along the process. Combining the results of gas velocity and slag Foaming Index, the evolution of the foam height could be determined. A maximum height was obtained in the first half of the blow, indicating that this part of the process is more susceptible to slopping events.
REFERENCES
1. Baptizmanskii V., V. Kulikov, B. Boichenko and E. Tretyakov E., "Improving slag formation in oxygen furnaces", Steel in the USSR, 8, 634-638, (1973).
2. Cicutti, C., M. Valdez, T. Pérez, R. Donayo, A. Gómez and J. Petroni, "Estudio de la evolución de la escoria y el baño metálico en el convertidor", 12º Seminario de Acería IAS, Buenos Aires, 629-638, (1999).
3. Cicutti C., M. Valdez, T. Pérez, J. Petroni, A. Gómez, R. Donayo and L. Ferro, "Study of slag-metal reactions in an LD-LBE converter", Sixth International Conference on Molten Slags, Fluxes and Salts, Stockholm-Helsinki, Paper 367, (2000).
4. Deo B. and R. Boom, Fundamentals of Steelmaking Metallurgy, Prentice Hall International, (1993).
5. Faure H., "Development, state of the art and future aspects of steelmaking", Revue de Metallurgie, 90, 1439-1449, (1993).
6. Ghag S., P. Hayes and H. Lee, "Physical model studies on slag foaming", ISIJ International, 38, N° 11, 1201-1207, (1998).
7. van Horn A., J. van Konynenburg and P. Kreyger, "Evolution of slag composition and weight during the blow" Mc Master Symposium Nº7: The role of slags in Basic Oxygen Steelmaking, 21-25, (1976).
8. Ito K. and R. Fruehan, "Study on the foaming of CaO-SiO2-FeO slags. Part I. Foaming parameters and experimental results", Metallurgical Transactions B, 20 B, 509-514, (1988a).
9. Ito K. and R. Fruehan, "Study on the foaming of CaO-SiO2-FeO slags. Part II. Dimensional analysis and foaming in iron and steelmaking processes", Metallurgical Transactions B, 20 B, 515-521 (1988b).
10. Kreijer P. and R. Boom, "Slag formation in large scale BOF steelmaking", Canadian Metallurgical Quarterly, 21, Nº 4, 339-345, (1982).
11. Margot-Marette H. and P. Riboud, "Fusibilité et cristallisation des laitiers basiques peu phosphatés d´aciérie", Revue de Metallurgie, 61, 709-716, (1964).
12. Mills K. and B. Keene, "Physical properties of BOS slags", International Materials Review, 32, Nº 1-2, 1-120, (1987).
13. Riboud P., Y. Roux, L. Lucas and H. Gaye, "Improvements of continuous casting powders", Fachber. Hüttenprax. Metallweit., 19, 859-869, (1981).
14. Slag Atlas, Ed. by Verein Deutscher Eisenhüttenleute, 2nd Edition, (1995).
15. Takawa T., K. Katayama, K. Katohgi and T. Kuribayashi, "Analysis of converter process variables from exhaust gas", Transactions ISIJ, 28, 59-67 (1988).
16. Traebert A., M. Modigell, P. Monheim and K. Hack, "Development of a modelling technique for non-equilibrium metallurgical processes", Scandinavian Journal of Metallurgy, 28, 285-290, (1999).
17. Turkdogan E., Physicochemical Properties of Molten Slags and Glasses, The Metals Society, (1983).
18. Turkdogan E., Fundamentals of Steelmaking, The Institute of materials, (1996).
19. Utigard T. and M. Zamaolla, "Foam behaviour in liquid FeO-CaO-SiO2 slags", Scandinavian Journal of Metallurgy, 22, 83-90, (1993).
20. Wu K., Chu S., S. Qian and Q. Niu, "Investigation into rheological characteristic and foaming behaviour of molten slags", Steel Research, 70, N° 7, 248-251, (1999) [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ]
Received: March 15, 2001.
Accepted for publication: December 15, 2001.
Recommended by Subject Editor E. Dvorkin.














