Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Latin American applied research
versão impressa ISSN 0327-0793versão On-line ISSN 1851-8796
Lat. Am. appl. res. v.32 n.4 Bahía Blanca dez. 2002
Localized corrosion of zirconium and zircaloy-4 in iodine alcoholic solutions
S. B. Farina §, G. S. Duffo §,* and J. R. Galvele §,*
§ Comisión Nacional de Energía Atómica, Depto. Materiales, Av. Gral. Paz 1499, (1650) San Martín, Buenos Aires, Argentina farina@cnea.gov.ar
* CONICET
Abstract&—The stress corrosion cracking (SCC) susceptibility of zirconium and one of its alloys, Zircaloy-4, was studied in 10 g/L iodine dissolved in various alcohols: methanol, ethanol, 1-propanol, 1-butanol and 1-pentanol. SCC was observed in all the systems studied and the crack propagation rate was found to vary depending on the size of the solvent molecule. When the molecular weight of the solvent molecule increased, the crack propagation rate decreased. Intergranular attack was also found in all the solutions tested, and the rate of intergranular corrosion also varied with the size of the solvent molecule. This behavior is attributed to a steric effect, which hinders the access of the corrosive species to the tip of the crack. The results found agree with the predictions of the surface mobility-SCC mechanism.
Keywords&— Zircaloy-4. Zirconium. Stress corrosion cracking. Pitting. Intergranular attack.
I. INTRODUCTION
Zirconium alloys are used in power nuclear reactors as fuel element cladding and also as structural material. The fuel element cladding is susceptible to stress corrosion cracking (SCC) induced by the iodine liberated during the fission of uranium (Cox, 1973). On the other hand, the structural components undergo embrittlement mainly due to the precipitation of zirconium hydrides.
Numerous authors have studied the SCC of Zircaloy-4 (Zry-4) in iodine vapor at 300ºC simulating the conditions existing in a nuclear reactor and the results were reviewed by Cox (1990). However, some authors have shown that this phenomenon can be simulated in the laboratory at low temperatures using solutions of iodine in methanol (Schuster and Lemaignan, 1989; Cox, 1977). Elayaperumal et al. (1972/3) studied the SCC of Zircaloy-2 in boiling methanolic solutions of iodine and they found that the susceptibility to the phenomenon increased with the iodine concentration, but that it was independent of the applied electrochemical potential. Cox (1975), who studied the SCC susceptibility of zirconium alloys in iodine solutions in various organic solvents, found that two groups of solvents could be distinguished: those in which SCC only takes place on precracked specimens, and those in which SCC also takes place on smooth specimens. Cox attributed this difference to the degree of stability of the complex formed between the iodine and the solvents, no reference being made to the effect of the size of the solvent molecules.
On the other hand, Mori et al. (1966) studied the SCC of Zr and Ti in HCl dissolved in three different alcohols, and determined that the higher the molecular weight of the alcohol molecule used, the higher the time to fracture of the specimens. From this work the possibility arises that the SCC susceptibility of zirconium and its alloys in iodine solutions depends on the size of the solvent molecule employed. For this reason in the present work, the SCC susceptibility of Zr and Zry-4 was systematically studied in solutions of iodine in several alcohols, at room temperature. Also the possible existence of other types of localized corrosion was considered.
II. EXPERIMENTAL METHOD
The samples used were 1 mm diameter wires of Zr and Zry-4. The chemical compositions (in w/o) are shown in Table 1.
The specimens were degreased in boiling ethylic ether and dried with hot air. Then, they were annealed in argon atmosphere (240 mm Hg) for 48 h at 800ºC in the case of Zr, and for 2 h at 750ºC in the case of Zry-4. In both cases the annealing was made in quartz tubes, with samples wrapped in tantalum sheets to avoid the contamination with silicon. After annealing, the samples were furnace cooled for 24 hours. Prior to the tests, the wires were degreased with acetone and dried with hot air.
Table 1. Chemical compositions (in w/o) of the materials used in the present work
| Element | Zirconium | Zircaloy-4 |
| Fe | 0.06 | 0.25±0.01 |
| Cr | 0.01 | 0.13±0.01 |
| Sn | <0.01 | 1.74±0.01 |
| Si | <0.0025 | <0.002D |
| B | <0.00004 | <0.002ND |
| Bi |
| <0.002ND |
| Co | <0.0015 | <0.02ND |
| Cu | 0.0025 | »0.05 |
| Mg | <0.001 | <0.0005D |
| Ni | <0.004 | <0.0005ND |
| Mo | <0.0025 | <0.0005ND |
| Ti | 0.0025 | <0.0005ND |
| V | <0.003 | <0.02ND |
| O | 0.1 | 0.13 |
| H | 0.0025 | 0.0025 |
| Zr | balance | balance |
ND: not detected, D: detected
The experiments were carried out in solutions of iodine in the following alcohols: methanol, ethanol, 1-propanol, 1-butanol and 1-pentanol. In all cases the concentration of iodine was 10 g/L and the solutions were prepared with analytical grade reagents. All the experiments were performed at room temperature. The constant elongation rate tests were carried out using a conventional glass cell described in a previous paper (Galvele et al., 1974), at the corrosion potential and at an initial strain rate of 4.7x10-6 s–1. The wires were strained to fracture. After fracture, the specimens were observed under a scanning electron microscope (Philips SEM 500) and then metallographically mounted and sectioned in order to measure the crack depth. The mean crack propagation rate was calculated by using two alternative methods. One was based on the SEM observation, the length of the brittle zone was measured on the fracture surface, and this value was divided by the straining time. The other method was based on the metallographic observation. The length of the longest crack was divided by the straining time. For the sake of comparison, wire samples of Zr and Zry–4 were also strained to rupture in air and in each of the pure alcohols, at room temperature and at the same straining rate.
In order to determine the existence of intergranular attack, and to measure its propagation rate, approximately 2 cm long wire samples of both Zr and Zry-4 were exposed to the iodine alcoholic solutions. The exposition time varied from 1 hour to 300 hours. Then the wires were metallographically mounted and sectioned in order to measure, in case of existing, the attack depth.
A. Intergranular Attack
Both Zr and Zry-4 were found to undergo intergranular attack in all the iodine alcoholic solutions studied. Figure 1 shows the depth of the intergranular attack for Zr as a function of the exposure time in the various iodine alcoholic solutions. The longest penetration depth was observed in the iodine in methanol solution, where, after a 20 hour exposure, the attack reached the diameter of the wire. In the iodine ethanol solution the attack rate decreased sharply and this tendency continued as the size of the solvent molecules increased. The results obtained in iodine in propanol are located between those of iodine in ethanol and those of iodine in butanol, but they are not shown in the figure for the sake of simplicity.
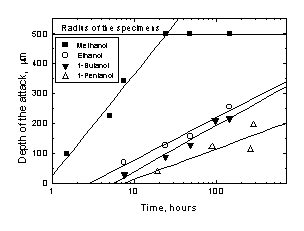
Figure 1. Intergranular attack depth for Zr as a function of the exposure time in iodine solutions in various alcohols.
Although in Zry-4 the rate at which the attack advanced was slightly lower than that of Zr, the general trend was similar. The iodine in methanol solutions was the most aggressive. After a 100 hour exposure, the attack reached the diameter of the wire. In the iodine in ethanol solution, the rate of the attack was lower and this tendency continued with the greater alcohol molecules.
B. Slow strain rate tests
Wire samples of Zr were strained to rupture at the rest potential and with an initial strain rate of 4.7x10-6 s-1 in pure alcohol, in the iodine alcoholic solutions and in air. The elongation to rupture in the various pure alcohols was found to be inside the dispersion band measured in air, which ranged from 14% up to 19%. The fracture surfaces in these cases showed no brittle zones. In the iodine alcoholic solutions, on the other hand, a sharp decrease in the deformation to rupture was observed. The fracture surfaces showed a typical brittle area and numerous lateral cracks were also observed, revealing the existence of SCC. Figure 2 shows the measured deformation to rupture for the various solutions tested.
In the iodine in methanol solution the deformation to rupture decreased to values ranging between 0.2% and 0.3%, this solution being the most aggressive from the SCC point of view. In the iodine in ethanol solution the deformation to rupture was of the order of 2%.
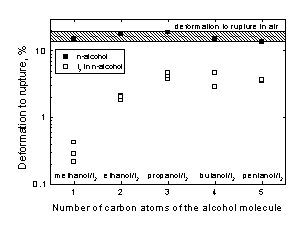
Figure 2. Deformation to rupture of Zr strained in various iodine alcoholic solutions.
In general, it was found that when the number of carbon atoms of the alcohol molecule increased (that is to say, when the size of the alcohol molecule increased), the deformation to rupture came closer to the values obtained in air, indicating a lower susceptibility to SCC.
In the case of Zry-4 the tests were also carried out in pure alcohol, in iodine alcoholic solutions and in air. It was found that the deformation to rupture in the pure alcohols was inside the dispersion band of the results obtained in air, which ranged from 15% to 20%. In the iodine alcoholic solutions, on the other hand, the decrease in the deformation to rupture, together with the appearance of a brittle fracture surface and numerous lateral cracks, showed the existence of SCC. The iodine in methanol solution, where the deformation to rupture decreased to values of approximately 1%, was the most aggressive one from a SCC point of view. Again, as in the case of Zr, it was found that when increasing the number of carbon atoms of the solvent molecule, the SCC susceptibility, measured through the elongation to rupture, decreased.
On the lateral surfaces of the samples strained in the iodine in methanol solution, besides the cracks, severe attack of the grain boundaries was also observed and in many specimens even some grains were missing (Fig. 3).
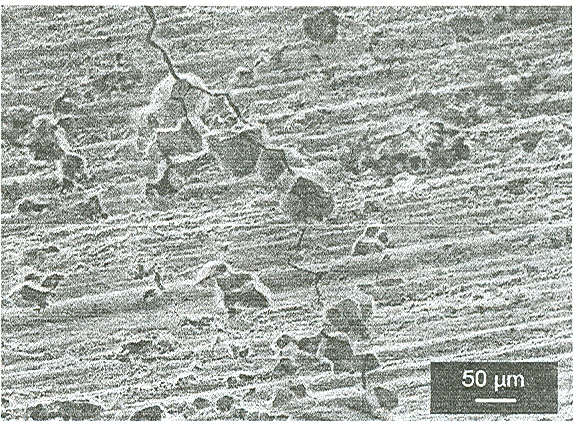
Figure 3. Wire of Zr strained to rupture in iodine + methanol solution (360x).
In the case of the samples strained in the iodine in ethanol solution, besides cracks, the existence of pits of various sizes evenly distributed along the wires were observed. The lateral surfaces of the specimens strained in the solutions of iodine in 1-propanol, in 1-butanol and in 1‑pentanol showed widespread attack besides numerous cracks. In all cases studies (in Zr, in Zry-4, and in all the iodine alcoholic solutions tested) the fracture surfaces were mixed. The cracks started with a zone of intergranular attack, followed by a zone of transgranular fracture plus a ductile fracture zone (Fig. 4 and Fig. 5).
The length of both the intergranular (IG) and the transgranular (TG) zones was measured. The intergranular penetration was found to decrease when the size of the solvent molecule increased. On the other hand, the length of the transgranular zone was almost constant and therefore, the total length of the cracks (intergranular plus transgranular zone) decreased when the number of carbon atoms of the alcohol molecule increased. The results obtained for Zr are shown in Fig. 6. Similar results were found for Zry-4.

Figure 4. Fracture surface of a specimen strained to rupture in a solution of iodine in methanol (200x).

Figure 5. Transition zone between IG and TG cracking morphology for Zr in a solution of iodine in 1-propanol (720x).
According to some authors (Schuster and Lemaignan, 1989) the intergranular-transgranular transition takes place when a critical value of the stress intensity (KI) is reached. The value of KI is related to the stress at the tip of the crack (s) and the length of the crack (a) through the expression:
KI µ s.a0.5 (1)
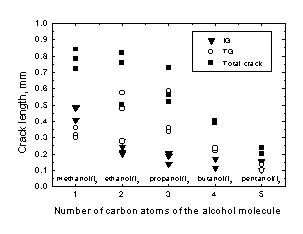
Figure 6. Length of the intergranular (IG), transgranular (TG) and total crack obtained for Zr strained in the iodine alcoholic solutions.
In the case of iodine in methanol solutions, the straining tests lasted only a few minutes, so that s was not very high, but since the rate of intergranular attack in these media was high, the intergranular penetration was important and the critical value of KI was reached with high values of a and low values of s. The opposite case happened in the solutions of iodine in 1-pentanol, where the rate of intergranular attack was low, and the tests lasted 2 or 3 hours allowing high values of s at the tip of the crack. In this latter case, the critical KI was obtained with high s values and small crack lengths. This is the reason why in the methanol solution an extensive region of intergranular attack was found before the transition to transgranular cracking, while in the 1-pentanol solution, the intergranular region was much smaller.
The total length of the brittle area (intergranular plus transgranular) measured on the fracture surface divided by the fracture time provided the crack propagation rate (cpr1). As it was mentioned above, an alternative way of calculating the crack propagation rate was to measure the length of the longest crack and dividing it by the fracture time (cpr2). Whenever possible (that is to say, when lateral cracks existed) both measurements were made. The values of cpr found for the case of Zr are shown in Fig. 7. Although the values of cpr1 are always higher than those of cpr2 (since cpr1 is calculated from the length of the crack that actually caused the fracture of the specimen and it is, of course, longer than any lateral crack), both results followed the same trend. The greatest cpr values (of the order of 10-6 m/s) were obtained in solutions of iodine in methanol. A smooth decrease in the cpr was observed when going from methanol to 1‑pentanol, that is to say, when the size of the solvent molecule was increased. Similar results were found for Zry-4.
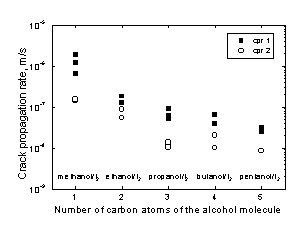
Figure 7. Crack propagation rates (cpr) for Zr in all the iodine solutions tested.
According to the surface mobility-SCC mechanism (Galvele, 1987), environmentally induced crack propagation is due to the capture of vacancies by the stressed lattice at the tip of the crack. The rate controlling step is the rate of movement of the vacancies along the surfaces of the crack, and the role of the environment is to change the surface self-diffusivity of the metal. It is known that low melting point contaminants formed on the surface increase the surface mobility and therefore enhanced SCC. In the present case, the low melting point compound formed is ZrI4 and hence surface-mobility-SCC should be expected. But, due to a problem of steric hindrance caused by the solvent molecule, the iodine molecules have difficulty in arriving at the tip of the crack to form the low melting point compound and therefore lower cpr values than those predicted by the mechanism are observed.
IV. CONCLUSIONS
The following conclusions can be drawn from the present paper:
• Zr and Zry-4 are susceptible to SCC in iodine alcoholic solutions at room temperature and at the rest potential.
• The SCC susceptibility, measured through the percentage of deformation to rupture and the cpr, is related to the size of the solvent molecule. This behavior seems to be associated with a problem of steric hindrance, since when increasing the size of the alcohol molecule, the SCC susceptibility decreases.
• It was also found that both materials undergo intergranular attack and, in agreement with a problem of steric hindrance the rate of the attack decreases as the size of the solvent molecule increases.
• The surface mobility SCC mechanism accounted for the experimental observations made in the present work.
ACKNOWLEDGMENTS
The financial support of the CONICET, and of the FONCYT, Secretaría de Ciencia y Tecnología, from Argentina, is acknowledged.
REFERENCES
Cox, B., Stress corrosion cracking of Zircaloys in iodine containing environments, Zirconium in Nuclear Applications, ASTM Special Technical Publication 551, 419-434 (1973). [ Links ]
Cox, B., Environmentally induced cracking of zirconium alloys, Reviews on Coatings and Corrosion 1 (4), 367-422 (1975). [ Links ]
Cox, B., Transient species participating in the SCC of zirconium alloys, Corros. 33 (3), 79-84 (1977). [ Links ]
Cox, B., Environmentally induced cracking of zirconium alloys-A review, J. Nucl. Mat. 170, 1-23 (1990). [ Links ]
Elayaperumal, K., P. K. De and J. Balachandra, Stress-corrosion cracking of Zircaloy-2 in methanol-iodine solutions, J. Nucl. Mater 45, 323-330 (1972/73). [ Links ]
Galvele, J. R., S. M. de De Micheli, I. L. Muller, S. B. de Wexler and I. L. Alanis, Localized Corrosion (Eds. R. W. Staehle, B. F. Brown, J. Kruger and A. Agrawal), NACE, Houston, 590-600 (1974). [ Links ]
Galvele, J. R., A stress corrosion cracking mechanism based on surface mobility Corros. Sci. 27 (1), 1-33 (1987). [ Links ]
Mori, K., A. Takamura and T. Shimose, Stress corrosion cracking of Ti and Zr in HCl-methanol solutions, Corros. 22 (2), 29-31 (1966). [ Links ]
Schuster, I. and C. Lemaignan, Characterisation of Zircaloy corrosion fatigue phenomena in an iodine environment, J. Nucl. Mat. 166, 348-356 (1989). [ Links ]
Received: May 11, 2001.
Accepted for publication: June 7, 2002.
Reccomended by Subject Editor A. L. Cukierman and Guest Editors E. L. Tavani and J. E. Perez Ipiña














