Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Latin American applied research
Print version ISSN 0327-0793On-line version ISSN 1851-8796
Lat. Am. appl. res. vol.32 no.4 Bahía Blanca Dec. 2002
The role of acidithiobacillus caldus in the bioleaching of metal sulfides
M. Semenza, M. Viera, G. Curutchet and E. Donati
Centro de Investigación y Desarrollo de Fermentaciones Industriales (CINDEFI-CONICET), Facultad de Ciencias Exactas (UNLP), 1900 La Plata, Argentina donati@quimica.unlp.edu.ar
Abstract&— In the absence of iron, dissolution of zinc sulfide was enhanced by the action of Acidithiobacillus caldus at 40oC. The bioleaching mechanism was similar to that observed for mesophilic species of Acidithiobacillus at 30oC, although the final metal recovery was lower. When iron was added to the cultures, the solubilization of zinc and copper from the sulfides was higher than that in sterile controls. The activity of the cells was through two indirect mechanisms (acid and oxidant mechanisms, for zinc and copper sulfides respectively). A. caldus did not enhance the dissolution of nickel sulfide neither in the absence nor in the presence of iron.
Keywords&— Metal sulfides. Acidithiobacillus caldus. Bioleaching. Sulfur.
Acidithiobacillus ferrooxidans (A. ferrooxidans) and Acidithiobacillus thiooxidans (A. thiooxidans) are considered the most important microorganisms in the bacterial dissolution of metal sulfides (bioleaching). These two species of Acidithiobacillus are gram-negative, aerobic and chemoautotrophic organisms. They develop at ambient temperatures (mesophilic bacteria) and are common in acid-polluted environments. Both bacteria are able to grow using energy obtained from reduced sulfur compounds. A. ferrooxidans is also capable of oxidizing iron(II) using oxygen as the last electron acceptor (Barrett et al., 1993; Rawlings, 1997). Since the discovery of bacterial leaching, two different mechanisms have been proposed to explain bacterial attack by A. ferrooxidans: a direct one and an indirect one. The direct mechanism is based on catalytic sulfide oxidation, while the indirect one implies sulfide oxidation by ferric ions producing sulfur and ferrous ions. These products are oxidized by the microorganisms allowing the iron redox cycle to be repeated (Rawlings, 1997; Donati et al., 1996).
Recently, two indirect leaching mechanisms have been proposed to explain degradation of sulfides. Both mechanisms combine characteristics of the former direct and indirect mechanisms. One is based on the oxidative attack of ferric iron on acid-insoluble metal sulfides involving thiosulfate as the main intermediate ( Schippers et al., 1996). The other mechanism is started by proton and/or ferric iron attack on acid-soluble metal sulfides with polysulfides and sulfur as intermediates ( Schippers and Sand, 1999).
Lately, another sulfur-oxidizing bacterium was found in continuous flow biooxidation tanks operating at temperatures between 40 and 50oC. This bacterium named Acidithiobacillus caldus (A. caldus) is a moderately thermophilic unable to oxidize iron(II) and it is a close relative of the mesophilic A. thiooxidans (Rawlings, 1997; Hallberg et al., 1996; Dopson and Lindstrom, 1999; Rawlings et al., 1999). A. caldus is also an aerobic, gram-negative and chemoautotrophic organism that generates energy from the oxidation of reduced sulfur compounds.
The fact that under certain conditions A. caldus can dominate the sulfur-oxidizing bacterial populations in commercial bioleaching and biooxidation plants, suggests that its role in the bioleaching of sulfides is more important than that recognized up to this moment. Studies on the bioleaching of sulfide ores have used mixed populations of A. caldus and iron-oxidizing bacteria (A. ferrooxidans or Leptospirillum. ferrooxidans) but pure population of A. caldus has not been used yet (Dopson and Lindstrom, 1999). In this paper, we have studied the role of a pure culture of A. caldus in metal sulfide bioleaching at moderately high temperature in the presence and in the absence of iron.
II. METHODS
A. Bacteria
A. caldus (ATCC 51756) was grown in batch culture at 40oC in a medium (Dopson and Lindstrom, 1999) consisting in the basal salts (g/l) (NH4)2SO4 (3.0), Na2SO4.10H2O (3.2), KCl (0.1), KH2PO4 (0.05), MgSO4.7H2O (0.5) including 1 % w/v elemental sulfur as energy source. The medium was adjusted to pH 2.5 with H2SO4. After removal of sulfur by filtration through blue ribbon filter paper (pore size 3 mm), cultures were centrifuged at 10000 g for 10 minutes and finally cells were suspended in the medium at pH 2.5. These suspensions were used as inocula in the leaching experiments. Bacterial population in these inocula was 1.0-2.7x108 cells/ml.
B. ExperimentsLeaching experiments were carried out in 500-ml flasks with 140 ml of medium (see above) inoculated with 10 ml of the bacterial suspension. Medium was previously sterilized by filtration through a 0.22-mm pore-size filter. In some experiments, 1 g/l Fe(II) as ferrous sulfate instead of sulfur, was added to the medium. Different pure sulfides (CuS, NiS and ZnS) were used at pulp densities of 0.10, 0.25 and 0.50 % weight/volume (w/v) in experiments without iron and 0.20 % in experiments with iron. The particle size was <200 mesh. The initial pH was 2.5 and it was not controlled throughout the experiments. Sterile controls were prepared replacing inocula by the same volume of sterile medium. Flasks were incubated in an orbital shaker at 180 rpm and at 40oC. All experiments were carried out at least in duplicate.
C. Analytical methodsIn periodic samples (previously filtered) the release of metal (zinc, copper and nickel) was followed by atomic absorption spectrophotometry. Sulfate concentration was monitored by a turbidimetric method (Vogel, 1978). Bacterial population in liquid phase was counted with a Petroff-Hausser chamber using a phase-contrasting microscope. Solid residues were analyzed using X-ray diffraction in order to detect compounds produced at the substrate surfaces during bioleaching.
III. RESULTS AND DISCUSSION
A. Experiments without iron
Figure 1 shows the results obtained when A. caldus was grown using sulfur as the sole energy source. The four parameters represented in this figure (proton and sulfate concentrations, pH and free bacterial population) show the evolution of the bacterial growth on elemental sulfur. In sterile controls there was not change in proton and sulfate concentrations (data not shown).
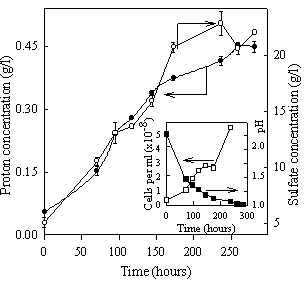
Figure 1. Bacterial growth on elemental sulfur. Proton and sulfate concentrations (outer graph). Free bacterial population and pH (inner graph).
The progress of proton concentration correlates with the corresponding evolution of sulfate concentration for the first 150 hours. During this period the ratio between proton and sulfate production was 2.8 which is higher than the stoichiometric ratio (mol H+/mol SO42-= 2) for sulfur oxidation according to the equation:
S + 3/2 O2 + H2O ®¾ H2SO4 (1)
This low sulfate production indicates that the mechanism of sulfur oxidation is similar to that accepted for other species of Acidithiobacillus. According to this mechanism, the first step of sulfur oxidation should generate protons and intermediate compounds such as sulfite and thiosulfate. In the last step of the sulfur oxidation, the intermediate compounds should be oxidized to sulfate with no significant proton production. Our results suggest that intermediate compounds are accumulated in the culture during the first 150 hours.
At the end of the growth the ratio between proton and sulfate concentration tends to reach the theoretical value. This could mean that cells oxidize mainly the intermediate compounds when sulfur begins to be the limiting nutrient.
This capability of producing sulfuric acid through the oxidation of sulfur shows the two roles of A. caldus during the bioleaching processes. One of them is the continuous acidification of the medium allowing the solubilization of metal compounds. The other role is the dissolution of the sulfur layer deposited on the sulfide during the bioleaching process, allowing further dissolution of the sulfides.
Figure 2 shows zinc concentration and free bacterial population versus time during the bioleaching of ZnS. Zinc extraction was higher in A. caldus culture than in the sterile control. It can be observed in the inner graph that the evolution of free bacterial populations has the same behavior than zinc extraction.
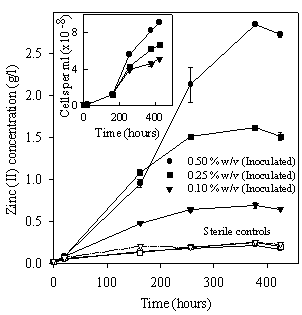
Figure 2. Bioleaching of zinc sulfide using A. caldus. Zinc concentration (outer graph) and free bacterial population (inner graph).
Figure 3 shows the percentages of metal recovery in the leaching of NiS and CuS after 15 days. As it can be seen, results of the chemical leaching experiments were similar to those for the bacterial leaching experiments.
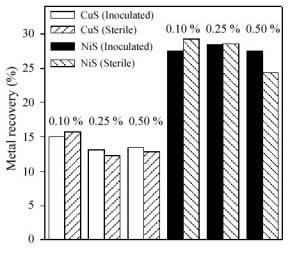
Figure 3. Percentages of metal recovery during the bioleaching of nickel and copper sulfides.
No significant bacterial action was detected in our experiments using A. caldus, except in the case of ZnS. This suggests a strong correlation between solubility of the sulfide and the efficiency of bioleaching. Thus, there was a high metal extraction in the case of the ZnS, which is more soluble than the other sulfides. Our results could be explained by the mechanism reported for A. thiooxidans (Pistorio et al., 1994). The first step of this mechanism is the chemical dissolution of the sulfide producing Zn2+ and H2S (Eqn. 2) followed by the bacterial oxidation of these species (Eqn. 3).
The removal of H2S allows the continuous dissolution of the sulfide due to the displacement of the equilibrium indicated by Eqn. 2. Sulfur formed in Eqn. 3 can be oxidized by cells according to Eqn. 1.
ZnS + 2 H+ ®¾ Zn2+ + H2S (2)
H2S + 1/2 O2 ®¾ S + H2O (3)
The behavior of pH (Figure 4) is in agreement with these processes: an initial slight increase of the pH (Eqn. 2) and a final decrease due to sulfur oxidation. In sterile controls the pH decrease was not observed and final pH was about 2.6 when the maximum yield of metal extraction (from 0.17 to 0.22 g/l according to the pulp density) was reached (Figure 2).
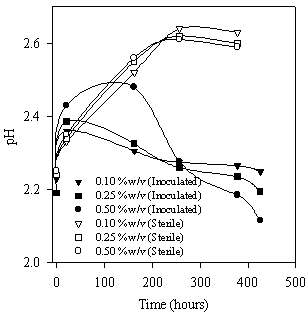
Figure 4. pH evolution during bioleaching of zinc sulfide using A. caldus cells.
Yields of zinc solubilization were 0.65 g/l (96 %), 1.51 g/l (90 %) and 2.73 g/l (81 %) and the rates of solubilization were 0.181, 0.103 and 0.044 g/l.day for 0.50, 0.25 and 0.10 % w/v pulp densities respectively. Although ZnS bioleaching was effective, the yields of zinc recovery and rates of zinc solubilization were lower than those obtained in cultures of A. ferrooxidans or A. thiooxidans at 30oC where the rates of solubilization were 0.233 and 0.270 g/l.day respectively at 0.20 % w/v pulp density (Pistorio et al., 1994). In A. caldus cultures, free bacterial populations reached higher values than those reported for other species of Acidithiobacillus. This suggests that A. caldus cells present less affinity for sulfur (Eqn. 1) reducing the number of attached cells. Because of that, the two roles of the bacteria are partially repressed and consequently the efficiency of metal recovery decreases significantly.
According to Fig. 3, it can be seen that there was not enhancement of nickel or copper dissolution in the presence of A. caldus cells. However, there was a small growth of the free bacterial population especially for NiS at the lowest pulp density (data not shown). A significant increase of pH could have inhibited the bacterial activity and the consequent sulfide dissolution. Similar behavior was observed in the case of A. thiooxidans although A. ferrooxidans enhanced significantly the copper solubilization (Porro et al., 1997); these results suggest that the mechanisms involved in the oxidation of very insoluble sulfides (as CuS) and moderately insoluble sulfides (as ZnS) are different ( Pogliani et al., 1990).
B. Experiments with ironIn other series of experiments, the effect of iron on the metal recoveries was analyzed. Figure 5 (outer graph) shows the evolution of zinc and copper concentration during leaching experiments. The inner graph shows suspended bacterial population in the cultures. Inoculation improved metal extraction from both pure sulfides (ZnS and CuS) reaching 0.99 g/l (73.5 %) and 0.92 g/l (69.1 %) of metal concentration respectively after 40 days. The rate of released zinc (0.0336 g/l.day) from the sulfide was much lower than those obtained in the absence of iron.
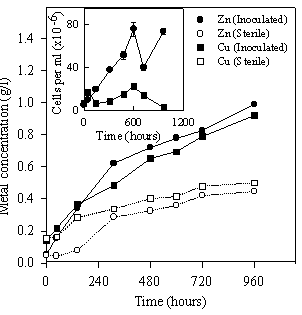
Figure 5. Zinc and copper concentration (outer graph) and free bacterial population (inner graph) during leaching experiments using A. caldus cells in the presence of iron.
In cultures and in sterile controls with zinc sulfide, a slight decrease of soluble iron was detected (data not shown). Soluble iron was mainly as iron (II). Thus, in agreement with experiments in the absence of iron, ZnS should have been solubilized by acid action. This process increased the pH (decreasing the solubilization rate) as it was found in sterile control where the final pH was 3.4. In cultures, there was not pH increase because A. caldus cells are able to oxidize H2S to H2SO4 allowing further acid action (data not shown). The sulfate production (19 mM) during the bioleaching agrees with the amount of solubilized zinc (15 mM) indicating that ZnS was solubilized according to the mechanism proposed in the absence of iron (Eqns. 2, 3, 1).
In the case of CuS there was not a significant pH change neither in the absence nor in the presence of bacteria. Because of this, CuS (a very insoluble sulfide) is slightly solubilized by acid action. However, ferric iron was detected in these systems; uncompleted ferrous iron oxidation was due exclusively to the action of air either in cultures or in sterile controls. In this way, CuS was oxidized by ferric iron producing sulfur and solubilizing copper (Eqn. 4).
CuS + 2 Fe3+ ®¾ S + Cu2+ + 2 Fe2+ (4)
A sulfur layer was formed on the substrate protecting it from further chemical oxidation. Finally, A. caldus dissolved the sulfur layer deposited on the sulfide surface allowing a further oxidation of CuS by ferric iron. This mechanism was confirmed by the difference between copper solubilized in cultures and in sterile controls (4.9 mM) which is very close to the difference between iron (II) concentration in the same systems (5.6 mM) and to the sulfate production in the cultures (about 4.6 mM). Moreover, elemental sulfur was detected by X-ray diffraction analysis in the solid residues from the sterile controls (but not from the cultures). Similar mechanism was previously observed for A. thiooxidans culture ( Curutchet et al., 1995;Pogliani and Donati, 2000). Bacterial population in suspension was lower than that in cultures with zinc sulfide; this agrees with the amount of sulfate produced in both cases. In these systems, we found a great iron decrease in solution (higher than 50 % of the initial amount). In solid residues from inoculated and sterile systems, jarosite, troilite (FeS) and pyrrhotite (Fe7S8) were also detected.
Results of the chemical and bacterial leaching of NiS were similar. In both systems, the decrease in iron concentration was almost equivalent to the increase of metal concentration. Although the mechanism has not been elucidated yet, a replacement of the metal (nickel) in the solid phase by iron was confirmed by the formation of phases as (Fe,Ni)9S8 and pyrrhotite and troilite. Another sulfide (Ni7S6) has also been found in the solid residues. Finally, jarosite deposits were also detected in the solid residues (the iron (III) precipitation was as high as in cultures with CuS).
Summarizing, this paper shows that in the absence of iron, A. caldus cells are only able to oxidize sulfur or to leach soluble sulfides as ZnS. In the presence of iron, the solubilization of zinc and copper from the sulfides was higher than that in sterile controls. During the leaching of ZnS, A. caldus enhanced zinc extraction through the oxidation of H2S produced for the acid action on the sulfide; moreover, the sulfur biooxidation did not allow the consequent increase of pH. In the case of CuS, there was a low oxidation of iron(II) by air. Iron(III) oxidized the sulfide producing sulfur which was deposited on the sulfide. The role of the cells was to dissolve the sulfur layer allowing further dissolution of the sulfide. These mechanisms are similar to those observed in cultures of A. thiooxidans although the solubilization rates detected in these last cultures were higher. Finally, A. caldus was unable to enhance the solubilization of NiS neither in the presence nor in the absence of iron.
E.Donati and G. Curutchet are research members of CONICET. Financial support from Agencia Nacional de Promoción Científica y Tecnológica is acknowledged.
1. Barrett, J., M.N. Hughes, G.I. Karavaiko and P.A. Spencer, Metal extraction by bacterial oxidation of minerals, Ellis Horwood, Chichester (1993). [ Links ]
2. Curutchet, G., C. Pogliani and E. Donati, Indirect bioleaching of covellite by Thiobacillus thiooxidans with an oxidant agent, Biotechnol. Lett. 17, 1251-1256 (1995). [ Links ]
3. Donati, E., G. Curutchet, C. Pogliani and P.H. Tedesco, Bioleaching of covellite using pure and mixed cultures of Thiobacillus ferrooxidans and Thiobacillus thiooxidans, Proc. Biochem. 31, 129-134 (1996). [ Links ]
4. Dopson, M.D. and E. B. Lindstrom, Potential role of Thiobacillus caldus in arsenopyrite bioleaching, Appl. Environ. Microbiol. 65, 36-40 (1999). [ Links ]
5. Hallberg K.H., M. Dopson and E.B. Lindstrom, Reduced sulfur compound oxidation by Thiobacillus caldus, J. Bacteriol. 178, 6-11 (1996) [ Links ]
6. Pistorio, M., G. Curutchet, E. Donati and P.H. Tedesco, Direct zinc sulphide bioleaching by Thiobacillus ferrooxidans and Thiobacillus thiooxidans, Biotechnol. Lett. 16, 419-424 (1994). [ Links ]
7. Pogliani, C., G. Curutchet, E. Donati and P.H. Tedesco, A need for direct contact with particle surfaces in the bacterial oxidation of covellite in the absence of a chemical lixiviant, Biotechnol. Lett. 12, 515-518 (1990) [ Links ]
8. Pogliani, C. and E. Donati, Enhancement of copper dissolution from a sulfide ore by using Thiobacillus thiooxidans, Geomicrobiol. J. 17, 35-42 (2000). [ Links ]
9. Porro, S., S. Ramírez, C. Reche, G. Curutchet, S. Alonso and E. Donati, Bacterial attachment: its role in bioleaching processes, Proc. Biochem. 32, 573-578 (1997). [ Links ]
10. Rawlings, D.E., Biomining: Theory, Microbes and Industrial Processes, Springer-Verlag, Berlin (1997). [ Links ]
11. Rawlings, D.E., N.J. Coram, M.N. Gardner and S.M. Deane, Thiobacillus caldus and Leptospirillum ferrooxidans are widely distributed in continuous flow biooxidation tanks used to treat a variety of metal containing ores and concentrates, In: Biohydrometallurgy and the Environment toward the Mining of the 21st Century, (Amils, R. and A. Ballester, Eds), Elsevier, Amsterdam, 777-786 (1999). [ Links ]
12. Schippers, A., P.G. Jozsa and W. Sand, Sulfur chemistry in bacterial leaching of pyrite, Appl. Environ. Microbiol. 62, 3424-3431 (1996). [ Links ]
13. Schippers, A. and W. Sand, Bacterial leaching of metal sulfides proceeds by two indirect mechanisms via thiosulfate or via polysulfides and sulfur, Appl. Environ. Microbiol. 65, 319-321 (1999). [ Links ]
14. Silverman, M.P. and D.G. Lundgren, Studies on the chemoautotrophic iron bacterium Thiobacillus ferrooxidans. I. An improved medium and a harvesting procedure for securing high cellular yields, J. Bacteriol. 77, 642-647 (1959). [ Links ]
15. Vogel, A.I., Textbook of Quantitative Inorganic Analysis 4th Ed., Basset, Denney, Mendham Revs. Longman, London (1978) [ Links ]
Received: May 11, 2001.
Accepted for publication: June 28, 2002.
Recommended by Subject Editor A. L. Cukierman and Guest Editors E. L. Taviani and J. E. Perez Ipiña














