Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Latin American applied research
versión impresa ISSN 0327-0793
Lat. Am. appl. res. v.33 n.1 Bahía Blanca ene./mar. 2003
Thermal effects of minority chalcogenide minerals: dta-tg, ir spectroscopy and sem electron microscopy studies
V. L. Barone1, I.L. Botto1 and I.B. Schalamuk2
1 Centro de Química Inorgánica (CEQUINOR-CONICET), Facultad de Ciencias Exactas, Universidad Nacional de La Plata, (1900) La Plata, Argentina. e-mail botto@quimica.unlp.edu.ar 2 Instituto de Recursos Minerales (INREMI-CIC), Facultad de Ciencias Naturales y Museo, Universidad Nacional de La Plata, (1900) La Plata, Argentina.
Abstract&— Aspects related to the thermal behaviour of minority chalcogenides containing Bi, Sb, Pb, Cu and Ag, associated with massive binary sulfides of economical importance, have been analyzed and compared. Selected samples proceed from different Argentine ore deposits. DTA-TG measurements of jamesonite (FePb4Sb6S14), eucairite (CuAgSe) and tetradymite (Bi2STe2 ) have been registered in oxidant atmosphere. The binary mineral chalcocite (Cu2S) has been also analyzed and employed with comparative purposes. The study was carried out with the aid of X-ray diffraction (XRD), IR spectroscopy and scanning electron microscopy (SEM) with microprobe chemical analysis (EDAX). Mass and energy changes during the thermal treatment can be associated with the increase of the metallic character of chalcogen and subsequently with the reactivity of the intermediate dioxide or oxocompound.
Keywords&— Chalcogenides, thermal behaviour, jamesonite, tetradymite, eucairite.
I. INTRODUCTION
The roasting process of metal sulfide to produce the metal is the basis of an important process used industrially on a large scale. Basically, it implicates an oxidation process to remove sulfur as volatile oxide.
So, over the centuries, lead and copper metals have been produced from galena and chalcopyrite, massive sulfide minerals, by this pyrometallurgical route. For long time, the extractive metallurgy of these metals has been directed towards energy economy (Habashi, 1985). In this respect, the presence of impurities originated by minority minerals namely sulfosalts and metal chalcogenides has been a problem very difficult to solve. This type of compounds, usually associated with common massive sulfides, affects
not only the energetic efficiency of the process but also the quality of the raw metals. In fact, it is well known that certain metalloids, e.g. antimony and bismuth, contaminate the copper and for this reason they must be removed previously by selective and expensive leaching processes. New technologies (e.g. hydrometallurgical route) have been developed in the last decades to reduce not only the dangerous evolution of SO2 and the undesirable presence of impurities but also the energetic cost of the process. In spite of these disadvantages, the thermal conversion of sulfide is even employed as extractive metallurgy for lead and copper.
Sulfosalts show a complex composition and structure (Makovicky, 1989) and the insufficient characterization of the starting materials in which these minority phases are included can affect the thermal reaction path. Hence, the assignment of the thermal effects of the massive sulfide can be made on a speculative basis. In the last years the characterization of the samples has been improved with a large amount of techniques (as spectroscopy and microscopy), which allow to assign more confidence to each thermal event (Dunn, 1997).
The oxidation occurs as a sequence of solid-gas and solid-solid reactions although it is usually controlled by the oxygen diffusion. This mechanism is observed as the first step. Thus, the evolution of chalcogenide dioxides with the temperature increase is related to the respective atomic weight.
The formation of oxocompounds followed by their decomposition as well as solid-solid and/or solid-gas reactions can be overlapped with ionic diffusional processes.
This work is a comparative analysis of the thermal oxidation of three minority species. We intend to demonstrate the behaviour of chalcogenides in the process. For this reason, we have selected the complex sulfide FePb4Sb6S14 (jamesonite), a selenide of composition CuAgSe (eucairite) and a sulfo-telluride of formula Bi2STe2 (tetradymite). Results are comparatively analyzed with respect to that of the binary species Cu2S (chalcocite) (Dunn et al., 1994). Qualitative and quantitative information about the progress of the oxidation as a function of temperature has been obtained by means of X-ray diffraction (XRD), IR spectroscopy and scanning electron microscopy (SEM-EDAX).
The thermal balance is associated with the presence of each chalcogenide, particularly the formation and properties of dioxides and oxocompounds, revealing the role that they can play from an industrial point of view.
II. EXPERIMENTAL
Mineral specimens of eucairite, jamesonite, tetradymite and chalcocite from different ore deposits of Argentina were selected by hand picking under microscope.
The monomineralogical composition of each species was checked by X-ray diffraction analysis (XRD) and scanning electron microscopy (SEM-EDAX). The particle size was smaller than 100 mm. The XRD analysis was carried out in a Philips PW 1714 equipment (CuKa radiation, Ni filter). DTA-TG experiments were performed on a Shimadzu thermoanalyzer DTA 50-TG 50 in an air atmosphere of 50 ml.min-1. Additional measurements in static air atmosphere were also carried out. All the thermoanalytical measurements were registered by using sample weights ranged between 20 and 50 mg. The heating rate of 10 °C.min -1. and alumina crucibles were used. The alumina (a-Al2O3) was employed as reference.
To analyze intermediate products, additional thermal studies were done with larger samples in a controlled temperature furnace in similar experimental conditions.
IR spectra were registered in a Perkin Elmer 580-B spectrophotometer (KBr pellet technique).
SEM measurements were performed in a Philips 505 electron microscope with an EDAX 9100 instrument.
III. RESULTS AND DISCUSSION
Typical TG and DTA curves for the chalcocite, eucairite, jamesonite and tetradymite are observed in Fig. 1. It is well known that the oxidation is always the initial exothermic process, which, in the most studied sulfides (Balá, et al. 1992 a), occurs through a partial formation of the metal oxide and/or the metal sulfate. The former reaction occurs with the simultaneous SO2 evolution (mass-loss and low temperature endothermic process) whereas the sulfate formation (associated with a mass-gain process) is followed by a desulfation step (mass-loss and high temperature endothermic process).
The metallurgical route depends on several experimental factors. Particle size, heating rate and atmosphere are particularly important and variations in these parameters can produce changes in the reaction mechanism.
On the other hand, in recent years the mechanical activation of inorganic substances seems to be a new factor that can be considered. This leads to metastable solids. The energy excess produced by grinding, accumulated in the solid, can be detected by thermal methods (Balá, et al. 1992 b). The reaction sequence is only valid for one set of conditions and is not valid for another conditions (Dunn, 1997). For this reason, the information obtained with well characterized starting material and under well controlled experimental conditions can be useful to design pilot plants and to produce significant savings in industrial operations.
The mass and thermal balance of some common sulfides (copper, iron, silver, molybdenum monometallic phases) can be schematically represented as:
2MS (s) + 3 O2 (g) Þ 2 MO (s) + 2 SO2 (g)
or MS (s)+ 2 O2 (g) Þ MSO4 (s)
followed by the sulfate decomposition according
to:
2 MSO4 (s) Þ MO.MSO4 (s) + SO3 (g)
MO.MSO4 (s) Þ 2 MO (s) + SO3 (g)
This process can be affected by the presence of chalcogenides or sulfosalts as impurities (Dunn, 1997). In this matter, the properties of the different non-metal dioxides, particularly the thermal stability, play a decisive role in the course of the process because the dioxide volatilization is ~700 °C for paratellurite (TeO2) and ~350 °C for SeO2. This contributes to the formation of intermediate mixed oxides by solid-solid reactions as the metallic character of the chalcogen increases.
DTA-TG plot of the binary sulfide chalcocite, one of the most important source of copper, observed in Fig. 1(a) shows an endothermic peak of phase transformation at low temperature (110 °C) and an exothermic signal from 400 °C, associated with a mass-gain, attributed to the sulfide oxidation. This process occurs through the formation of copper sulfate and Cu2O.

Figure 1. DTA (top)- TG (bottom) curves for
a) chalcocite, b) jamesonite, c) tetradymite,
d) eucairite.
The high temperature endothermic event is governed by the oxi-sulfate desulfation (CuO.CuSO4) and the Cu(I) oxidation with the production of CuO as unique final product. Our results are in agreement with reported data (Dunn, 1997).
The oxidation of the binary sulfide molybdenite (MoS2) is another interesting example of mineral roasting that leads to a unique oxide phase, in this case of prominent commercial importance (Shigegaki et al. 1988).
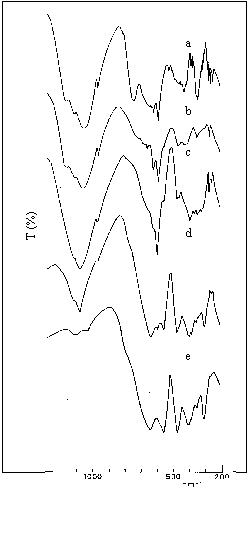
Figure 2. IR spectra of jamesonite by thermal treatment: a) 550 ºC, b) 650 ºC, c) 850 ºC, d) 1000 ºC, e) 1300 ºC.
The thermal behaviour of jamesonite (FePb4Sb6S14) is directed by an evident exothermic process but without appreciable mass change. The thermal reaction leads to the Sb2O3, FeSO4 and PbSO4 formation. The production of the last compound is associated with a low evolution of SO2. However, this gas assures the reducing atmosphere that prevents the Fe(III) presence. In fact, this effect has been corroborated by working in static air. The FeSO4 decomposes at ~ 650 °C, while the desulfation of PbSO4 occurs at higher temperature (850 °C).
The SO3 evolution generates the PbO and FeO oxides which by reaction at hight temperature form two mixed oxide phases: FeSb2O6 (rutile type) and Fe0.67Pb1.33Sb2O7 (pyrochlore type) according to our previous results by electron diffraction (Landa Canovas et al., 1993).
As it has been demonstrated in other works (Dunn, 1997), we have corroborated the importance of the IR spectroscopy to analyze the progress of the oxidation reaction.
The thermal route, analyzed by IR spectroscopy, is observed in Fig. 2.
Bands at ~1000 cm-1, attributed to the S-O antisymmetric stretching of tetrahedral sulfate, disappear as temperature increases, whereas the bands below 700 cm-1 are assigned to the mixed oxides.
The decomposition scheme in air atmosphere as temperature increases can be represented as:
FePb4Sb6S14Þ3Sb2O3 +FeSO4 + 4PbSO4 + 9SO2
ß (650 ºC)
FeO+ SO3ß (850 ºC)
4PbO+4SO3
ß (~1000 ºC)
0.33 FeSb2O6 + Fe0.67Pb1.33Sb2O7 + 1.67 Sb2O3 + 2.67 PbO
The tetradymite (Bi2STe2) presents a thermal behaviour associated with an exothermic process with a marked mass-gain (Botto et al., 1995).
The oxidation reaction starts at low temperature (~300 °C) with the formation of Bi2(SO4)3 and subsequently (BiO)2SO4 according to the IR spectra (680 cm-1 for the BiO+ bismuthyl species).
The TeO2 (paratellurite) presence was clearly observed from 450-500 °C by XRD and IR spectroscopy (bands below 800 cm-1). This oxide melts at ~ 600 °C and sublimates at ~700 °C.
However, part of TeO2 (~ 60 %) reacts with Bi2O3 to form a Bi1-XTeXO(1.5+X/2) solid solution, structurally related to the cubic dBi2O3 (non stoichiometric fluorite type) (Mercurio et al., 1991).
The phase with the Bi8Te3O18 composition, according to EDAX data, melts around 850 °C.

Figure 3. Micrographs of eucairite: a) original sample (x1000, 10 mm); b) heated at 600 ºC (x500, 100 mm); c) heated at 1100ºC (x5000; 10mm).
With the evidence collected for the tetradymite roasting in air atmosphere, the reaction sequence is:
4 Bi2STe2 Þ 1.35 Bi2(SO4)3 + 8TeO2 + 2.65 Bi2O3
ß (~400 °C)
1.35 (BiO)2SO4 + 2.65 SO3
ß (~600-800 °C)
~0.55 "Bi8Te9O18" + 1.35 SO3 + 5 TeO2
5.5 (Bi1.454Te0.546 O3. 273)
On the other hand, the eucairite CuAgSe shows a particular thermal behaviour with a marked mass-loss without signal of exothermic oxidation. This is surely due to the existence of two simultaneous and compensated energetic changes. In fact, the endothermic process of SeO2 evolution, immediate to its formation (from ~350 °C) agrees with the pronounced mass decrease. Below 200 °C a small endothermic peak of a phase transformation is observed (195 °C), while a first small mass-loss (~2 %) is attributed to the formation of a CuAgSe1-X non stoichiometric phase. The incipient formation of Ag2SeO3 and copper oxide in the solid residue is inferred by XRD and IR spectroscopy from ~350 oC.
Similarly, the formation of the unstable CuSeO3, in a first oxidation step, cannot be disregarded.
However, it is very difficult to observe the copper selenite because it decomposes at the temperature of the initial oxidation to give the intermediate and also unstable non stoichiometric product (2CuO.SeO2) containing up to 80 % of CuO excess (Verma, 1999).
The formation of Ag2SeO3 as intermediate product can be clearly observed at ~550-600 °C and the intensity of the XRD lines diminishes from this temperature.
Simultaneously, typical XRD lines of tenorite (CuO) and Ag (metal) were also observed, becoming progressively more intense as the temperature increases.
Micrographs of Fig. 3 show three steps of the treatment whereas the corresponding EDAX data reveal a gradual decrease of selenium content with the temperature.
Samples were not homogeneous at 600 °C, with variable Cu and Ag contents due to the coexistence of two or more phases. In fact, Ag values between 7.20 and 45.91 % correlated to Cu data of 82.59 to 21.74 % respectively were observed. Results show a trend in agreement with the Se disappearance from T~250 °C up to ~800 °C.
The diminution and subsequent increase of Ag content with temperature (20 % at 700 °C and 56 % at 900 °C) were associated with the progressive aggregate of thin particles of Ag metal that cropped out on the surface up to the melting point, forming so large drops.
All results permitted us to formulate the following scheme of eucairite decomposition:
2CuAgSe Þ 2AgCuSe1-X
(350 ºC) ß "CuSeO3" ?
Ag2SeO3 + 2 CuO + (1-2x) SeO2
(~600 ºC) ß
2 Agº + SeO2
IV. CONCLUSIONS
-Oxidative thermal decomposition of minority chalcogenides, associated with massive sulfides in economically ore deposits, allows to reveal thermal and mass changes of importance in the metallurgical route of common sulfides.
-The magnitude of the qualitative and quantitative changes depends on the sulfosalt or chalcogenide composition.
-It is possible to establish the sequence of intermediate reactions by means of the IR spectroscopy, XRD, and SEM-EDAX microscopy.
-The formation of non-metal dioxides, oxocompounds and/or mixed oxides as well as the oxide sublimation is revealed. So, thermal and mass balance can be related to the metallic character of the chalcogen and the thermal stability of intermediate oxocompound.
-A shrinking core model could be applied to represent the particle oxidation. It appears that oxygen attacks the outer surface and the reaction front moves inwards. In this solid-gas process the chalcogenide oxocompounds [sulphate (SO4=), selenite (SeO3=), tellurite (TeO3=) and oxides] seem to be simultaneously formed.
-The knowledge of the effect of impurities on the thermal behaviour of workable massive sulfides can produce significant savings in plant operations.
ACKNOWLEDGEMENTS
Authors thank CONICET, ANPCyT (Argentina) for financial support and Lic. M. Sánchez for technical assistance.
REFERENCES
Balá,P., H.J. Dunn, and H. Heegn, "Differential thermal analysis of mechanically activated sphalerite", Thermochim. Acta,194, 189-195 (1992 a). [ Links ]
Balá, P., E. Post and Z. Bastl, "Thermoanalytical study of mechanically activated cinnabar", Thermochim. Acta, 200, 371-377 (1992 b). [ Links ]
Botto, I.L., A.M. Ramis, I.B. Schalamuk and M.A. Sánchez, "Thermal decomposition of Bi2STe2 Tetradymite", Thermochim. Acta, 249, 325-333 (1995). [ Links ]
Dunn, J. G., "The oxidation of sulphide minerals", Thermochim. Acta, 300, 127-139 (1997). [ Links ]
Dunn, J.G., A.R. Ginting and B. OConnor, "A thermoanalytical study of the oxidation of chalcocite", J. Therm. Analysis, 41, 671-686 (1994). [ Links ]
Habashi, F., "Principles of extractive metallurgy", Vol. 3, Pyrometallurgy, Gordon & Breach Science Publishers, New York (1985). [ Links ]
Landa-Canovas, A.R., L.C. Otero Díaz, I.L. Botto and I.B. Schalamuk, "Electron microscopy study of the decomposition product at 1300ºC from jamesonite mineral", Solid State Ionics, 63-65, 301-306 (1993). [ Links ]
Makovicky, E., "Modular classification of sulphosalts", Neues Jahrbuch Miner. Abh., 160, 269-297 (1989). [ Links ]
Mercurio, D., B.H. Parry, B. Frit, G. Harburn, R.P. Williams and R.J.D. Tilley, "A series of infinitely adaptive phases in the Bi2O3-TeO2 system", J. Solid State Chemistry, 92, 449-459 (1991). [ Links ]
Shigegaki, Y., S.K. Basu, M. Wakihara and M. Taniguchi, "Thermal analysis and kinetics of oxidation of molybdenum sulfides", Journal of Thermal Analysis, 34, 1427-1440 (1988). [ Links ]
Verma, V.P., "A review of synthetic thermoanalytical, IR, Raman and X-ray studies on metal selenites", Thermochim. Acta, 327, 63-102 (1999). [ Links ]














