Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Latin American applied research
versión impresa ISSN 0327-0793
Lat. Am. appl. res. v.33 n.1 Bahía Blanca ene./mar. 2003
Determination of kinetic aspects from the non-isothermal chlorination of tungsten minerals
C.J. Menéndez a,b, E.L. Tavani b and E.J. Nolasco a
a Instituto de Investigaciones en Tecnología Química (INTEQUI) Universidad Nacional de San Luis. C.C. 290, (5700) San Luis, Argentina
b Centro de Tecnología de Recursos Minerales y Cerámica (CETMIC) Comisión de Investigaciones Científicas de la Provincia de Buenos Aires C.C. 49, (1897) M.B. Gonnet, Argentina
Abstract— The main kinetic aspects of the chlorination of tungsten minerals with chlorine and sulphur dioxide were analyzed from data obtained under non-isothermal conditions. The tungsten minerals studied were wolframite, scheelite and a scheelite-wolframite concentrate. The collected minerals were milled and then concentrated by means of gravity separation. Chlorinations were made in a vertical reactor with a static bed and upward flow of reactive gases using an empirical methodology that was established taking into account the most important physicochemical features of the system. The rate equation and the apparent activation energy for each tungsten mineral were determined by the integration method using a characteristic temperature to evaluate the temperature integral. An easy applicable criterion was used to determine the corresponding values of the characteristic temperature. Results obtained allowed to establish that the movement of an interface controls the wolframite chlorination and that the presence of non-volatile reaction products affects the chlorination of scheelite and of the scheelite-wolframite concentrate. An operative sequence was proposed to determine the temperature range (initial and final) in which the respective rate equation selected from non-isothermal data maintained its validity. Finally, the characteristic temperature was calculated by a frequently used route and results obtained were compared in each case.
Keywords Kinetic aspects, non-isothermal chlorination, tungsten, wolframite, scheelite.
I. INTRODUCTION
The determination of kinetic parameters under non-isothermal conditions is an alternative method of analysis that allows to obtain acceptable results in thermal decomposition reactions (phyllosilicates, metal carbonates, sulphates, nitrates, nitrites, oxyhalides, chromites, etc.). The decomposition evolution is determined in a unique experiment when the sample is exposed to a systematic and controlled variation of the reaction temperature. Parameters are calculated from the curve that shows the conversion degree as a function of the reaction temperature (Popescu and Segal, 1998; Brito et al., 1996; El-Awad and Mahfouz, 1989; Felix and Girgis, 1989; Aglietti et al., 1988; Bamford and Tipper, 1980; Vallet, 1972; Horowitz and Metzger, 1963). Some authors consider that kinetic parameters obtained from a single experiment are less reliable than those determined from multiple experiments (Gotor et al., 1998; Criado et al., 1987).
The main tungsten minerals are wolframite (FexMn1-xWO4) and scheelite (CaWO4). The wolframite consists of an isomorphous series of ferberite (FeWO4) and hübnerite (MnWO4) (Blackburn and Dennen, 1994). In the nature, wolframite and scheelite can be found separately or associated between them. When this natural association occurs, both minerals are distributed in an irregular way in each tungsten-bearing grain and their separation is difficult to be performed under favourable economic conditions. In our country, sufficient resources of associated tungsten minerals were found so as to be used at industrial scale. At the same time, scarce resources of minerals without association are known (Angelelli, 1984).
The chlorination is an alternative process to recover tungsten from its bearing minerals (Menéndez, 1999; Nolasco et al., 1991; Yih and Wang, 1979). The reaction starts with adsorption of chlorine on the surface of the reactive solid. Then, the exchange of lattice oxygen by chlorine and the respective desorption of oxygen into the gaseous phase are produced. The efficiency of the process may be improved if a reducing agent is used to combine with the oxygen released during the reaction. The sulphur dioxide showed a reducing behaviour acceptable in the tungsten recovery by chlorination (Menéndez, 1999).
The chlorination of the tungsten minerals using chlorine and sulphur dioxide involves several reactions from which volatile products (WO2Cl2, FeCl3 and S2) and non-volatile products (CaSO4, CaCl2, FeCl2 and FeS) are formed. Volatile reaction products are desorbed from the reaction interface and diffuse in opposite directions to reactive gases. Whereas, non-volatile products are deposited on the mineral that remains without reacting (residue) and the reactive gases must diffuse through this barrier to reach the reaction interface. The kinetic analysis under non-isothermal conditions is interesting since it is applied to a multiple diffusion that does not occur in the mentioned thermal decomposition reactions. Consequently, the aim of this work was to establish criteria to determine kinetic aspects from the non-isothermal chlorination of wolframite, scheelite and a scheelite-wolframite concentrate.
II. EXPERIMENTAL
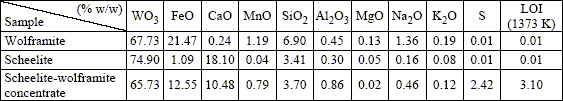
Tungsten minerals used come from San Martín, San Luis, Argentina. Three samples were collected containing low-grade wolframite, low-grade scheelite and a natural association of both minerals. Since studied resources are formed by systems of veins, it was necessary to carry out the milling of the samples in order to obtain the release of the valuable constituents. The concentration of the tungsten minerals was performed by gravity separation (jig and shaking table). The new samples obtained with this methodology were labelled as wolframite, scheelite and scheelite-wolframite concentrate. The chemical composition of these samples is indicated in Table 1.
The identification of minerals and reaction products was carried out by chemical analysis, X-ray diffraction (XRD) and scanning electron microscopy (SEM) with surface chemical composition determination.
Chemical analyses were made by titration and gravimetry for the major elements and by atomic absorption/emission (AA/AE) for the minor elements. The AA/AE analyses were performed with a Jarell Ash instrument. Analyses by XRD were carried out with a Philips 1140/00 equipment using a rotating sample holder and Cu Ka radiation (0.15405 nm). The SEM studies were made with a JEOL JSM-T100 scanning electron microscope. The surface chemical composition was determined by means of energy dispersive X-ray analysis with a Philips 505 scanning electron microscope and EDAX microanalysis system.
The chlorinations were made using chlorine as chlorinating agent and sulphur dioxide as reducing agent. The equipment utilized was composed of a vertical reactor, a premixing chamber of gases and three cylinders (one for chlorine, one for sulphur dioxide and other for nitrogen) with their corresponding control valves and flowmeters. Into the reactor, the sample was placed on a ceramic fiber (kaowool). Likewise, the use of this fiber permitted to improve the homogenization and the heating of the gaseous flow (reactive gases and inert gas). Each chlorination was carried out with 3 g of a new sample aliquot. This amount originated a bed thickness that did not present resistance to the passage of the gaseous flow. The tungsten extraction (a ) was determined by a destructive chemical analysis of the residue and each value reported in this work was the average of two chlorinations performed in the same way with a similar reproduction.
The reactor was heated by an electric furnace with temperature control. The working temperature was reached while a nitrogen flow circulated. When the system was stabilized at the working temperature, the nitrogen was replaced by the reactive gases maintaining the same flow rate during the time corresponding to each test. Then, the furnace was turned off and cooled down to reach the room temperature while nitrogen circulated again. The reaction temperature was determined through a thermocouple and displayed on a digital thermometer.
The fluidization and the formation of channels in the bed occurred when a gas flow rate of 1000 ml min-1 was employed. Both difficulties were negligible with a flow rate of 500 ml min-1. This chlorine flow rate during 1 min represents a reagent amount almost similar to the required by the reaction stoichiometry. Then, the use of a chlorine flow rate of 300 ml min-1 during 5 min assures a sufficient reagent excess. The flow was completed with 200 ml min-1 of sulphur dioxide employed as reducing agent in this work.
The kinetic analysis under non-isothermal conditions was made between 773 and 1173 K. The a values were determined as a function of the temperature for a constant reaction time of 5 min and constant flow rates of the reactive gases (300 ml min-1 of chlorine + 200 ml min-1 of sulphur dioxide). Figure 1 shows curves obtained for the non-isothermal chlorination of wolframite, scheelite and the scheelite-wolframite concentrate.
Table 1. Chemical analysis of tungsten minerals obtained by gravity separation.
III. RESULTS AND DISCUSSION
The concentration operations carried out in this work allowed to obtain three samples with similar tungsten content but with different mineral species. The main differences were that in the wolframite and scheelite samples they only had a unique and different tungsten mineral while the scheelite-wolframite concentrate had a natural association of both minerals (50-60% of scheelite and 25-35% of wolframite) and 7-14% of pyrite (FeS2). The presence of quartz and feldspars was determined in the three samples, although the contents of these impurities were different in each tungsten mineral.
The pyrite contained in the scheelite-wolframite concentrate decomposes when the furnace is heated to reach and to stabilize the working temperature. The decomposition occurs according to
2 FeS2 (s) ® 2 FeS (s) + S2 (g) (1)
Sulphur-bearing minerals still remaining in the reactor after stabilizing the working temperature (FeS2 and FeS) react with the oxygen released during the chlorination. The reaction (oxidation) of both substances is exothermic (Rosenqvist, 1987; Szczygiel Jordens and Torres Reyes, 1984), which originates a thermal gradient that was determined in tests carried out with the scheelite-wolframite concentrate. The chlorination temperature reached a maximum between 0.5-1.5 min and then decreased. After 2-3 min, the temperature was stabilized in its initial value. Residues so obtained did not have sulphur-bearing minerals. On the other hand, the thermal decomposition of the pyrite is favoured with the reaction temperature and time increase (Jovanovic, 1989). This decomposition changed the sulphur initial content of the scheelite-wolframite concentrate (the S2 condensed at the reactor exit), and therefore the thermal gradient decreased as the temperature and time of the tests increased.
The thermal gradient value also depended on the sample amount used in each test. Changes of the reaction temperature were not determined with 100 mg of sample. Nevertheless, such sample amount was considered as inadequate because the results so obtained have limited value (Habashi, 1992). Then, 3 g of scheelite-wolframite concentrate were used since this amount was the minimum one that allowed to maintain a constant ratio of the tungsten minerals and impurities. The same sample amount was also used for tests with wolframite and scheelite.

Figure 1. Tungsten extraction (a ) as a function of the temperature at t= 5 min and flow rates of 300 ml min-1 for chlorine and 200 ml min-1 for sulphur dioxide.
Tests performed with the scheelite-wolframite concentrate started at a lower temperature so as to reach the desired value with the thermal gradient. In order to compensate the decrease of the thermal gradient due to the exhaustion of pyrite, the temperature of the electric furnace was increased once the reaction began. The average of the maximum temperature and the final temperature was taken as the test temperature. Results were considered as acceptable when differences of both values were lower than 7 K.
The a values obtained at constant temperature for each tungsten mineral showed little changes after 5 min. According to this result, chlorinations were performed at 773, 823, 873, 923, 973, 1023, 1073, 1123 and 1173 K for 5 min. In each case, the volatile reaction products were condensed at the reactor exit while the non-volatile reaction products were deposited on the residue.
An identical tungsten-bearing volatile reaction product (WO2Cl2) was obtained from wolframite and scheelite. The wolframite chlorination also involved the FeCl2 formation (melting point= 950 K). Notwithstanding, the mentioned iron salt was not determined on the residues coming from tests carried out at temperatures lower than 950 K. This situation was attributed to the fact that the FeCl2 was transformed to FeCl3 (boiling point= 588 K) by the oxidizing action of chlorine (Nagata and Bolsaitis, 1987). In the upper part of the reactor where volatile reaction products were collected, the gaseous flow temperature decreased at ~573 K. The FeCl3 solidifies at 580 K and at this temperature its partial thermal decomposition to FeCl2 and Cl2 can also occur (McArdle, 1981). The presence of both iron salts was determined at the reactor exit, which confirmed that the iron (II) transformation is partially reversible. Accordingly, all products formed into the reactor were volatile
FeWO4 (s) + 2.5 Cl2 (g) + 2 SO2 (g) ® WO2Cl2 (g) + FeCl3 (g) + 2 SO3 (g) (2)
The sulphur trioxide was identified in the gases at the reactor exit (these gases were bubbled in Ba(OH)2 solution). The manganese participation in the reaction was not considered because it is found in small amount.
In the scheelite chlorination, besides the WO2Cl2 above mentioned, CaSO4 (melting point= 1723 K) and CaCl2 (melting point= 1055 K) were also formed. The CaCl2 presence was scarce since its formation is more difficult than the one of the CaSO4 under the operative conditions used by us (Menéndez, 1999). Then, we can assume that the reaction occurred according to
CaWO4 (s) + Cl2 (g) + SO2 (g) ® WO2Cl2 (g) + CaSO4 (s) (3)
The main differences in the scheelite-wolframite concentrate chlorination were originated by the occurrence of a thermal gradient and by the fact that the CaCl2 formation was favoured by the pyrite presence. This change of reaction products would be produced because the released oxygen during chlorination reacts in a larger amount with pyrite than with sulphur dioxide. The pyrite oxidation at low temperatures is performed with abundant amount of oxygen (2FeS2 (s) + 5.5 O2 (g) ® Fe2O3 (s) + 4 SO2 (g)), so we consider that the subsequent SO2 conversion to the sulphate species is not complete. Then, the neutralization of the Ca2+ cation coming from the scheelite is obtained by means of the CaCl2 formation (chemical analysis, XRD, SEM, EDAX). On the other hand, the chlorination temperature had a different effect on the reactivity of wolframite and scheelite. Thus, at high temperatures (1123 and 1173 K) the wolframite reacted almost completely while the scheelite reaction was substantially lower (XRD).
The weight loss is a non-destructive analysis that allows to determine the conversion degree in thermal decomposition reactions under non-isothermal conditions. These transformations are carried out with only one sample and the operative conditions are obtained through a continuous and simultaneous change of the reaction temperature and time. In our case, a discontinuous variation (systematic and controlled) of the temperature was made while the other variables were maintained constant (Bamford and Tipper, 1980).
The heterogeneity of the volatile and non-volatile reaction products formed during the chlorination impeded the determination of the a values by the mentioned non-destructive analysis. The a values were determined by means of an analytical technique that requires to finish the chemical attack of the residue (destructive chemical analysis), and consequently a new mineral aliquot was used to make each chlorination (Menéndez, 1999). Whereas, the scheelite-wolframite concentrate chlorination was performed so as to correct the temperature variations originated by the pyrite presence. Briefly, the procedure proposed by us to value the behaviour of each tungsten mineral involved changes to the methodology used in thermal decomposition reactions.
The rate of a chemical reaction can be interpreted with the differential equation
da /dt= k(T(t))f(a (t)) (4)
where a is the conversion degree after time t, f(a ) is the differential rate equation that depends on the reaction mechanism and k is the rate constant of the process. The k is related with the absolute temperature (T) by the Arrhenius equation
k= Aexp(-E/RT) (5)
where A is the frequency factor, E is the apparent activation energy and R is the gas constant. For a constant heating rate (b = dT/dt), combining equations, rearranging terms and integrating, the following is obtained
ò da /f(a )= (1/b )ò kdT= (A/b )ò exp(-E/RT)dT (6)
The evaluation of the temperature integral (right side) can be made by using a characteristic temperature (Tc) (Menéndez, 1999; Popescu and Segal, 1998; Aglietti et al., 1988; Bamford and Tipper, 1980; Vallet, 1972; Horowitz and Metzger, 1963), so that the difference between the temperature of each test (T) and the Tc selected fulfills with the inequality: ½ T-Tc½ < < Tc. After introducing the notation ò da /f(a )= g(a ), resolving the integral according to that proposed by Horowitz and Metzger (1963) and taking logarithms, the following simplified expression can be obtained
lng(a )= lng(a c) + (E/RTc2)(T-Tc) (7)
where g(a ) is the integral rate equation (used in the isothermal method) that depends on the reaction mechanism. The lng(a ) versus (T-Tc) plot is a straight line if the selection of g(a ) is correct and the E value is obtained from the slope (E/RTc2) corresponding to this straight line. The frequency factor and the heating rate are included in the term lng(ac).
In thermal decomposition reactions, the Tc value changes with the nature of the reaction products formed. When all the reaction products are volatile (as in the pyrolysis of polymers), Tc is defined as the temperature at which 1-a = 1/e, where e= 2.71828. For reactions in which the weight loss is a fraction of the total weight (as in the pyrolysis of hydrated salts), a method generally used is to choose Tc where da /dT is maximum. Summarizing, the reaction corresponding to each system starts and is finished within a temperature narrow range, the Tc value is defined according to the reaction type and the temperature inequality is easy to be fulfilled.
The Tc definition for our system is not so simple as in the cases of polymers and of hydrated salts. This fact occurs because the reaction products formed during the chlorination were heterogeneous and the tungsten extractions were obtained within a wide temperature range (Fig. 1). The average of the extreme temperatures (initial and final) that fulfills the integral rate equation is a value that may be used to obtain an acceptable approximation of the temperature inequality (½ T-Tc½ < < Tc).
The kinetic analysis was started considering as extreme temperatures 773 and 1173 K (Tc= 973 K). Although these temperatures did not fulfill adequately the mentioned inequality, g(a ) was selected from Eqn. (7). The linearity of each lng(a ) versus (T-Tc) plot was evaluated by the least square method. The difference existent among some kinetic models was not significant and consequently it was considered as appropriate to continue the analysis using as new extreme temperatures 823 and 1173 K (Tc= 998 K). With these extreme temperatures, the difference among kinetic models was sufficient to identify the respective rate equations. Table 2 shows the E and r2 values calculated from the rate equation selected for each tungsten mineral.
The best agreement found for the wolframite was given by the rate equation g(a )= 1-(1-a )1/2, which indicates that during the chlorination a non-uniform area contraction of the reactive particle is produced. At the same time, rate equations selected for the scheelite (g(a )= (1-a )ln(1-a ) + a ) and for the scheelite-wolframite concentrate (g(a )= (1-(1-a )1/3)2) showed that the diffusion of the reactive gases through the respective layers of the non-volatile reaction products was the stage that controlled the total kinetics.
In order to explain the different behaviour of the scheelite and the scheelite-wolframite concentrate, those aspects that affect the diffusion process were analyzed. The simplest diffusion mechanism is produced when the surface area is maintained almost constant and the reaction rate decreases as the thickness of a layer (barrier) of non-volatile reaction products deposited on the residue increases (Bamford and Tipper, 1980).
The scheelite and the scheelite-wolframite concentrate were observed by SEM before and after chlorinations. Thus, it was possible to determine that after the chlorination larger changes in the shape and size of the tungsten-bearing grains of the scheelite-wolframite concentrate were produced with respect to the scheelite. The analysis of the surface chemical composition (EDAX) before chlorination allowed to establish that the calcium distribution on each tungsten-bearing grain was more homogeneous in the scheelite. The pyrite presence in the scheelite-wolframite concentrate led to a higher CaCl2 formation instead of CaSO4. On the other hand, the lower calcium content in the scheelite-wolframite concentrate determined that for the attainment of the same tungsten extraction larger amount of calcium sulphate in the scheelite must be formed. Aspects analyzed above show that the composition and the thickness of each layer (barrier) were different. This would explain the fact that for describing the diffusion process an equation for the scheelite and other for the scheelite-wolframite concentrate were determined.
The tungsten extractions obtained at low temperatures from the scheelite-wolframite concentrate were not connected with those obtained from wolframite and scheelite (Fig. 1). Two mixtures were prepared to analyze this behaviour: one with scheelite and wolframite and the other with scheelite, wolframite and pyrite maintaining the same ratio that each mineral had in the scheelite-wolframite concentrate. Both samples were chlorinated under the same operative conditions at 873 K. The tungsten extraction from the mixture with pyrite was double than the extraction from the mixture without pyrite. The pyrite (present only in the scheelite-wolframite concentrate) is almost stable at 873 K, it has a high reducing potential with respect to the sulphur dioxide (reducing agent used in this work) and it determines a partial change of the non-volatile reaction products formed. These facts favour the yield of the reaction and would allow to interpret the obtained results.
The validity of the kinetic aspects above determined depends on that k does not change its function with the temperature (Levenspiel, 1996; Urbanovici and Segal, 1987). This behaviour may be evaluated from the slope corresponding to the relationship between lnk and 1/T (Eqn. (5)). The k value for each temperature can be estimated from the equation
g(a )= kt (8)
Equation (8) was obtained by integration of the rate equation expressed in differential form (Eqn. (4)). After replacing the k value according to Eqn. (5) and taking logarithms, it is observed that for the slope (behaviour) determination of the system it is not necessary to consider the lnt and lnA values
lng(a )= lnt + lnA - E/RT (9)
The lng(a ) versus 1/T plot for each mineral (Fig. 2) was determined from its respective rate equation and tungsten extractions obtained between 823 and 1173 K. The scheelite-wolframite concentrate plot showed a reasonable linearity in all the temperature range studied (r2= 0.9884). Whereas, wolframite and scheelite plots had an important dispersion at 1/T= 1.215 x 10-3 K-1 (T= 823 K). The exclusion of such value improved the linearity of both plots (r2 wolframite= 0.9777 and r2 scheelite= 0.9796). The kinetic analyses were repeated using as new extreme temperatures 873 and 1173 K (Tc= 1023 K), which favoured the fulfillment of the temperature inequality. Results so obtained showed that the reaction mechanisms determined for the three tungsten minerals were equal and that minimum changes in their apparent activation energies were produced (Table 2).

Figure 2. Evolution of the apparent activation energy with the temperature for the three tungsten minerals.
Finally, the Tc value was determined where da /dT is the maximum for each plot of Fig. 1. Evaluations were carried out with Microcal Origin 5.0 from tungsten extractions obtained between 873 and 1173 K (extreme temperatures that fulfilled the respective rate equations previously determined). The tungsten extraction plots for wolframite and for scheelite did not present a maximum while the scheelite-wolframite concentrate plot had a maximum at 1013 K (the evaluation made according to our proposal led to a value of Tc= 1023 K). Both Tc values for the concentrate were similar with the two evaluation routes used by us. Meanwhile, the absence of a maximum for scheelite and for wolframite explains the importance of having an alternative route to calculate Tc.
Table 2. Apparent activation energy for the non-isothermal chlorination of each tungsten mineral used in this work.

IV. CONCLUSIONS
The heterogeneity of the reaction products formed during the chlorination of wolframite, scheelite and the scheelite-wolframite concentrate impeded the determination of the tungsten extraction by weight loss. This determination was performed by means of a destructive chemical analysis using a new mineral aliquot for each test.
The pyrite is a reactive mineral whose presence was established only in the scheelite-wolframite concentrate. The pyrite thermal decomposition occurred while the working temperature was reached and so the sulphur initial content in this tungsten-bearing mineral decreased. The oxygen released during the chlorination reacted with the sulphur that still remained in the reactor, which determined a reaction temperature increase. The occurrence of this increase was attributed to the exothermic nature of the sulphur oxidation present in the pyrite. Taking into account these facts, an empirical methodology of heating was implemented and used in the chlorination of the scheelite-wolframite concentrate to correct the temperature variations originated by the pyrite presence.
The integration method allowed to determine conveniently the rate equation and the apparent activation energy for each tungsten mineral. The evaluation of the temperature integral was carried out using a characteristic temperature. The value of this temperature was obtained by two routes, one as the average of the initial and final temperatures that fulfilled the integral rate equation and other as the temperature at which the maximum tungsten extraction was obtained. The characteristic temperature was determined by both routes only for the scheelite-wolframite concentrate since tungsten extraction plots for wolframite and for scheelite did not present a maximum in the temperature range studied. The Tc values so obtained for the scheelite-wolframite concentrate had a reasonable agreement.
The rate equation that showed higher linearity for the non-isothermal chlorination of wolframite corresponded to the kinetic model of non-uniform area contraction of the reactive particle. In the cases of the scheelite and the scheelite-wolframite concentrate, the total kinetics was controlled by the diffusion of the reactive gases through the layer (barrier) of non-volatile reaction products deposited on the corresponding residue. Aspects analyzed indicated that each layer deposited was unequal. This inequality would allow to explain that for describing the diffusion process a rate equation for scheelite and other for scheelite-wolframite concentrate were determined.
An operative sequence established by us was used to confirm that the rate equation selected for each mineral maintained its validity between 873 and 1173 K. This behaviour was evaluated from the Arrhenius equation, the respective rate equation and the tungsten extractions obtained under non-isothermal conditions for wolframite, scheelite and scheelite-wolframite concentrate.
REFERENCES
Aglietti, E.F., H.J. Gasalla and J.M. Porto López, "Kinetics and compensation effect related to crystallinity in non-isothermal dehydroxylation of kaolinite", Lat. Am. Appl. Res., 18, 43-48 (1988). [ Links ]
Angelelli, V., Yacimientos metalíferos de la República Argentina, Comisión de Investigaciones Científicas de la Provincia de Buenos Aires, La Plata (1984). [ Links ]
Bamford, C.H. and C.F.H. Tipper, Comprehensive chemical kinetics, Elsevier, Amsterdam (1980). [ Links ]
Blackburn, W.H. and W.H. Dennen, Principles of mineralogy, Wm. C. Brown Publishers, Dubuque (1994). [ Links ]
Brito, Z., V. Torrealba, G. Sánchez and L. Hernández, "Methodology for kinetic characterization in compound materials from TGA curves, using digital image processing", Lat. Am. appl. res., 26S, 31-34 (1996). [ Links ]
Criado, J.M., A. Ortega and C. Real, "Mechanism of the thermal decomposition of anhydrous nickel nitrate", React. Solids, 4, 93-103 (1987). [ Links ]
El-Awad, A.M. and R.M. Mahfouz, "Kinetic analysis of isothermal, non-isothermal and catalysed thermal decomposition of malonic acid", J. Thermal Anal., 35, 1413-1421 (1989). [ Links ]
Felix, N.S. and B.S. Girgis, "Isothermal and nonisothermal gravimetry of nontronite dehydroxylation", J. Thermal Anal., 35, 743-750 (1989). [ Links ]
Gotor, F.J., M. Macías, A. Ortega and J.M. Criado, "Simultaneous use of isothermal, non-isothermal, and constant rate thermal analysis (CRTA) for discerning the kinetics of the thermal dissociation of smithsonite", Int. J. Chem. Kinet., 30, 647-655 (1998). [ Links ]
Habashi, F., Observations and remarks on research and development activities, Visit to Research Institutes of Argentina, San Luis (1992). [ Links ]
Horowitz, H.H. and G. Metzger, "A new analysis of thermogravimetric traces", Anal. Chem., 35, 1464-1468 (1963). [ Links ]
Jovanovic, D., "Kinetics of thermal decomposition of pyrite in an inert atmosphere", J. Thermal Anal., 35, 1483-1492 (1989). [ Links ]
Levenspiel, O., Ingeniería de las reacciones químicas, Editorial Reverté, México (1996). [ Links ]
McArdle, J.V., Iron compounds, Kirk-Othmer: Encyclopedia of Chemical Technology, Vol. 13, 3rd edition, M. Grayson Editor, John Wiley & Sons, New York (1981). [ Links ]
Menéndez, C.J., Estudio del proceso de cloración de minerales de tungsteno empleando una mezcla de cloro y dióxido de azufre, Tesis Doctoral, Universidad Nacional de San Luis (1999). [ Links ]
Nagata, K. and P. Bolsaitis, "Selective removal of iron oxide from laterite by sulphurization and chlorination", Int. J. Miner. Process., 19, 157-172 (1987). [ Links ]
Nolasco, E.J., O.D. Quiroga and J.B.P. Rivarola, "Estudio de la reacción entre scheelita y cloro en presencia de carbón", Rev. Metal. CENIM, 27, 102-108 (1991). [ Links ]
Popescu, C. and E. Segal, "Critical considerations on the methods for evaluating kinetic parameters from nonisothermal experiments", Int. J. Chem. Kinet., 30, 313-327 (1998). [ Links ]
Rosenqvist, T., Fundamentos de metalurgia extractiva, Editorial Limusa, México (1987). [ Links ]
Szczygiel Jordens, Z. and A. Torres Reyes, Metalurgia no ferrosa, Editorial Limusa, México (1984). [ Links ]
Urbanovici, E. and E. Segal, "Some problems concerning the mathematical theory of non-isothermal kinetics. I. Reactions described by rate equations of the form da /dt= Ak(T) f(a )", Termochim. Acta, 111, 335-348 (1987). [ Links ]
Vallet, P., Thermogravimétrie, Gauthier-Villars, Paris (1972). [ Links ]
Yih, S.W.H. and C.T. Wang, Tungsten: sources, metallurgy, properties and applications, Plenum Press, New York (1979). [ Links ]
Received: May 11, 2001.
Accepted for publication: August 23, 2002.
Recommended by Subject Editor A. L. Cukierman and Guest Editor J. E. Perez Ipiña














