Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Latin American applied research
versão impressa ISSN 0327-0793
Lat. Am. appl. res. v.33 n.1 Bahía Blanca jan./mar. 2003
Fusion characteristics of austenitic stainless steel gmaw welds
J. Lozanoa/b, P. Moredaa, C. L. Llorentea/b and P. D. Bilmesa
a Laboratorio de Investigaciones de Metalurgia Física (LIMF)-Facultad de Ingeniería-UNLP. Calle 1 y 47, 1900 La Plata, jolozano_ar@yahoo.com, pabilmes@volta.ing.unlp.edu.ar b LEMIT-CICPBA, cllorent@volta.ing.unlp.edu.ar
Abstract— Shielding gas is a key element in GMAW (Gas Metal Arc Welding). It represents only a small percentage of the overall production cost, and its proper choice makes possible to obtain well shaped and faultless beads, increasing both productivity and quality and therefore resulting cost saving. The influence of several shielding gases on the fusion characteristics of GMAW using solid wires of austenitic stainless steels is assessed in this work. Beads on plate welds are performed on AISI 304 steel plates using ER 308LSi welding wire according to AWS A5.9 and the following shielding gases: Ar, Ar-O2, Ar-CO2, Ar-He-CO2, He-Ar-CO2, Ar-CO2-NO, Ar-NO, Ar-He-H2, Ar-He. For these gas mixtures, stability and geometry of the weld are evaluated, determining depth of penetration, bead reinforcement height, width of the bead, wetting angle, fusion angle, total fusion area, plate fusion area and dilution. A comparative analysis of the results which are obtained with the different gas mixtures is carried out.
Keywords— Welding, stainless steel, shielding gases, GMAW process.
I. INTRODUCTION
GMAW has become an efficient manufacturing method for producing many types of welded structures. The reasons for the rapid development of GMAW are associated with high productivity, flexibility, and automation potential (Svensson and Elvander, 1999).
Today, the users of this process have started to realize that the shielding gas is not simply a component of the welding process. It is a "key" element in the three fold welding process: power source – material – shielding gas.
It is well known that the shielding gas, which represents about 3% of the total cost, has a noticeable effect on the GMAW features. According to Irving (1999), a proper choice of the shielding gas may lead to increased productivity and quality, as well as considerable cost saving through the production of well-shaped faultless beads.
II. EXPERIMENTAL PROCEDURE
"Bead on plate" GMAW welds were performed by using different shielding gases. The components of the different gas mixtures which were used in this work are shown in Table 1.
AISI 304 steel plates (80 x 200 x 6.4 mm) were used as base material. As a filler metal, a 1.2 mm solid wire of ER 308LSi, was used according to AWS A5.9. Chemical compositions of the base material and the solid wire are shown in Table 2.
A direct current power source was used to perform the bead on plate welds by means of automatic GMAW process. Welding parameters were chosen in order to obtain spray transfer mode for all shielding gases. These parameters were recorded and monitored through specific software for welding and they are shown in Table 3.
Table 1. Chemical composition of shielding gases.
| Gas | Components (%) |
| A* | Ar:81+He:18+CO2:1 |
| B** | Ar:98+O2:2 |
| C** | Ar:43+He:55+CO2:2 |
| D** | Ar:98+CO2:2 |
| E* | Ar:100 |
| F* | Ar:96+CO2:3+H2:1 |
| G*1 | Ar:95+He:5 |
| I* | Ar:98+O2:2 |
| J*2 | Ar: 99.97+NO:0.03 |
| K*2 | Ar:97.97+NO:0.03+CO2:2 |
| L* | Ar:78+He:20+CO2:2 |
2 NO is added for stabilizing the arc and reducing the ozone in working environment.
Table 2. Chemical composition of base material and solid wire.
| C | Mn | Si | Cr | |
| Base Material | 0.042 | 1.55 | 0.43 | 18.33 |
| Solid Wire | 0.010 | 1.80 | 0.80 | 19.65 |
| Ni | Mo | S | P | |
| Base Material | 8.11 | 0.39 | 0.019 | 0.014 |
| Solid Wire | 10.71 | 0.60 | 0.014 | 0.014 |
Three weld beads were performed and analyzed with each gas mixture under identical experimental conditions.
Cross sections of "bead on plate" welds, which were obtained with the different shielding gases, were prepared for macroscopic analysis. For revealing macrostructure, according to Beraha (1970), a solution based on HCl and K2S2O5 was applied.
Optic microscopy and a soft imaging system were used to analyze fusion characteristics of the welds, defined by Jeffersons Welding Encyclopedia (1997): width of the bead, bead reinforcement height, depth of penetration, wetting and fusion angles, reinforcement and penetration areas and dilution (Fig. 1).
Weld bead profile was also recorded, and X-ray tests were performed to evaluate discontinuities.

S: Reinforcement P: Penetration A: Width
Dilution% = B/ (C+B) x 100
B: Penetration area C: Reinforcement area
Figure 1. Fusion characteristics of weld beads.
Table 3. Welding parameters.
| Gas/Bead | Current | Voltage | Gas flow |
| A/1 | 215 | 29.5 | 20 |
| B/2 | 215 | 28.7 | 20 |
| C/3 | 220 | 30 | 20 |
| D/4 | 215 | 27 | 20 |
| E/5 | 220 | 29.8 | 20 |
| F/6 | 215 | 29.2 | 20 |
| G/7 | 225 | 28.8 | 20 |
| I/8 | 215 | 27 | 20 |
| J/9 | 215 | 27 | 20 |
| K/10 | 205 | 28 | 20 |
| L/11 | 190 | 31 | 20 |
| Gas/Bead | Welding speed | Contact tip-to-work distance | Heat input |
| A/1 | 40 | 1.5 | 9.37 |
| B/2 | 40 | 1.5 | 9.65 |
| C/3 | 40 | 1.5 | 9.75 |
| D/4 | 40 | 1.5 | 8.58 |
| E/5 | 40 | 1.5 | 9.68 |
| F/6 | 40 | 1.5 | 9.27 |
| G/7 | 40 | 1.5 | 9.57 |
| I/8 | 40 | 1.5 | 8.57 |
| J/9 | 40 | 1.5 | 8.57 |
| K/10 | 40 | 1.5 | 8.47 |
| L/11 | 40 | 1.5 | 8.70 |
III. RESULTS
Figure 2 shows the weld bead profiles which were obtained with the different shielding gases. The weld geometry parameters of the different welds are shown in Table 4. The fusion characteristics of the beads are in agreement with those reported in ANSI/AWS C5.6-94R (1994). In all cases, metal transfer was spray mode.
Figure 3 shows a voltage and current record corresponding to the test carried out with the "C" (high helium content) shielding gas.

Figure 2. Macrographs of weld beads obtained with different shielding gases.

Figure 3. Current and voltage record corresponding to the performed test with "C" shielding gas.
Table 4. Geometrical parameters of the different weld beads.
| Gas | Width (mm) | Penetration (mm) | Reinforcement height (mm) | Wetting angle |
| A | 11.51 | 2.71 | 2.42 | 38 |
| B | 10.6 | 3.29 | 2.56 | 30 |
| C | 15.57 | 4.2 | 2.9 | 12 |
| D | 12.67 | 3.38 | 2.9 | 27 |
| E | 15.67 | 3.1 | 2.13 | 33 |
| F | 12.42 | 3.88 | 2.64 | 24 |
| G | 14.75 | 3.73 | 2.17 | 29 |
| I | 10.05 | 3.29 | 2.56 | 36 |
| J | 10.63 | 3.28 | 2.66 | 35 |
| K | 10.16 | 3.75 | 2.97 | 38 |
| L | 11.81 | 2.7 | 2.42 | 46 |
| Gas | Fusion angle | Penetration area (mm2) | Reinforcement area (mm2) | Dilution (%) |
| A | 71 | 11.01 | 19.18 | 36.5 |
| B | 78 | 13.27 | 17.71 | 42.8 |
| C | 90 | 21.23 | 27.34 | 43.7 |
| D | 76 | 15.26 | 23.17 | 39.7 |
| E | 62 | 14.3 | 21.72 | 39.7 |
| F | 79 | 18.37 | 20.98 | 46.7 |
| G | 40 | 16.1 | 20.86 | 43.6 |
| I | 74 | 13.95 | 17.71 | 44.1 |
| J | 60 | 13.87 | 18.1 | 43.4 |
| K | 81 | 14.48 | 19.65 | 42.4 |
| L | 57 | 18.7 | 15.39 | 54.9 |
IV. DISCUSSION
Regarding discontinuities, there has been no evidence of internal and/or superficial irregularities, such as spatter, undercutting, overlapping or porosity in the beads which have been obtained with the different shielding gases, except for those obtained with "E" and "L" gases. For this last weld, the beads show undercutting, spatter, and a bad superficial shape. On the other hand, the bead which has been obtained with the "E" gas shows a marked overlapping and undercutting. Ar based gas mixtures have evidenced a better arc stability than high helium content gas mixtures.
Weld geometry parameters such as width of the bead, bead reinforcement height, and depth of penetration for welds which are obtained with the different gas mixtures are shown in Figs. 4 and 5. Correlating bead width and penetration, it can be seen that the highest values for width of the bead and penetration have been obtained with "C" and "G" gases (with high and low helium contents). On the other hand, the highest values for bead reinforcement have been mainly related to argon based gases ("K" and "D").

Figure 4. Bead width for each of shielding gases.

Figure 5. Bead reinforcement height and penetration for each of the shielding gases.
Regarding width of the beads, it can be observed that the widest beads have been obtained with helium gases ("C" and "G").
Ar based gases with low CO2 content, with and without hydrogen ("D" and "F" gases), come next on a decreasing scale. Even though one of the widest beads has been obtained using argon ("E" gas) it is not considered because of the overlapping it shows.
Bead reinforcement and penetration areas, as well as dilution, which are obtained with different gas mixtures, are shown in Figs. 6 and 7. Figure 8 shows wetting and fusion angles for each bead.

Figure 6. Penetration and reinforcement areas for each of the shielding gases

Figure 7. Dilution for each of the shielding gases

Figure 8. Wetting and fusion angles for each of the shielding gases
As the fusion angle increases in relation to deeper penetration, a better-shaped bead is obtained. In this sense, the best results have been obtained with "C", "F", and "K" gas mixtures (Fig. 9).
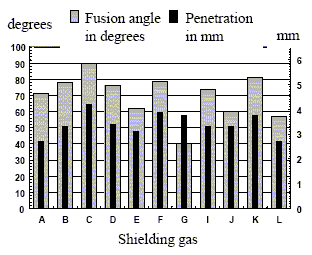
Figure 9. Fusion angle and penetration for each of the shielding gases
The bead produced with the high helium content ("C" gas mixture) has shown the highest penetration and fusion angle values. Although this bead has the largest reinforcement area, its dilution remains within the average for the rest of the beads.
From the results obtained with "D" (Ar+2%CO2) and "K" shielding gases (the latter being identical to "D" gas but with a 0.03%NO added) a tendency in favor of "K" gas is observed as it shows a smaller reinforcement area with greater dilution. Besides, it presents a better- shaped bead by means of a larger fusion angle and deeper penetration.
V. CONCLUSIONS
The widest bead and the deepest penetration have been achieved with "C" and "G", Ar+55%He+2%CO2 and Ar+5%He gas mixtures, respectively.
Argon based gases with low CO2 content: "K" and "D" with and without NO respectively, have shown the highest bead reinforcement height.
By increasing fusion angle and penetration, a better-shaped bead has been obtained. In this sense, "C", "F", and "K" gas mixtures have shown the best results.
The best shaped bead, with a low reinforcement height and greater width, together with a deeper penetration and high dilution, has been achieved with "C" gas which has a high helium content.
The addition of O2 and CO2 to argon produces better results than pure argon. However equivalent values to those obtained with high helium content gases have not been achieved.
Regarding fusion characteristics, small quantities of NO added to Ar-CO2 mixture and to pure argon would seem to improve the weld bead profile.
REFERENCES
ANSI/AWS C5.6-94R, "Recommended practices for gas metal arc welding", American Welding Society, Miami, U.S.A. (1994). [ Links ]
Beraha, E. "Metallographic reagents based on sulfide films", Prakt. Metalogr., 7, 242-248 (1970). [ Links ]
Irving, B., "Shielding gases are the key to innovations in welding", Welding Journal, 78 (1), 37-41 (1999). [ Links ]
Jeffersons Welding Encyclopedia, 8th Edition, American Welding Society, Miami, U.S.A. (1997). [ Links ]
Svensson, L.E. and J. Elvander, "Challenges for welding consumables for the new millennium", Svetsaren, 78, 3-11 (1999). [ Links ]
Received: May 11, 2001.
Accepted for publication: August 23, 2002.
Recommended by Subject Editor A. L. Cukierman and Guest Editors E. L. Tavani and J. E. Perez Ipiña














