Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Latin American applied research
versión impresa ISSN 0327-0793
Lat. Am. appl. res. v.33 n.2 Bahía Blanca abr./jun. 2003
Kinetic parameters for thermal inactivation of cut green beans lipoxigenase using unsteady-state methods
R. L. Garrote, E. R. Silva and R. A. Bertone
Instituto de Tecnología de Alimentos (ITA) -FIQ-UNL
Ciudad Universitaria, C.C. Nº 266- 3000 Santa Fe - Argentina
E-mail: rgarrote@fiqus.unl.edu.ar
Abstract- Most raw vegetables can be stored for only a short time even at -20ºC. Blanching is the primary pre-freezing means of inactivating undesirable enzymes present in the vegetable. Lipoxygenase is widely distributed in vegetables and evidence is mounting to support its involvement in off-flavour development and colour loss. In order to optimize the blanching process of vegetables, it is essential to establish a kinetic model of the inactivation of the indicator enzyme. Isothermal and dynamic thermal approaches have been used to determine kinetic parameters. The unsteady-state procedure is more flexible and can be applied to uniform and non-uniform heating situations and generally a food medium, rather than an aqueous buffer solution, is always used for determining kinetic parameters. Kinetic parameters describing lipoxygenase inactivation during heating of cut green beans were determined using two unsteady-state procedures. The model used an analytical solution for heat conduction in a finite cylinder to predict time-temperature profiles, and a trial and error and a nonlinear regression of experimental lipoxygenase retentions to estimate kinetic parameters, rate constant, k and activations energy, Ea. Thermal diffusivity, a , and convective heat transfer coefficient, h, were experimentally determined, but thermal conductivity, l , was estimated. Mean values obtained, with standard deviations between parenthesis, were k76ºC = 27.2 (9.4) s-1, k82ºC = 92.9 (7.5) s-1; k88ºC = 212.1 (52.7) s-1; k94ºC = 407.8 (56.7) s-1; Ea = 160.7 (8.1) KJ/mol using the trial and error procedure; k85ºC = 150 (26.3) s-1 and Ea = 164 (4.7) KJ/mol using the nonlinear regression method. Predicted and observed lipoxygenase retentions showed good agreement.
Keywords- Blanching Index. Heating Treatment and Enzymatic Activity.
I. INTRODUCTION
The blanching process involves exposing plant tissue to some form of heat, usually steam or hot water, for a prescribed time at a specified temperature. As a pre-freezing operation, blanching is the primary means of inactivating undesirable enzymes present in the vegetable (Barrett and Theerakulkait, 1995). The temperature-time combination used in the blanching process will generally be determined by the thermal stability of enzymes involved in quality deterioration of the processed product (Svensson and Ericksson, 1974b). Williams et al. (1986) evaluated the sensory character of blanched vegetable purées to which isolated enzymes had been added and found that lipoxygenase was the enzyme most active in aroma deterioration in English green peas and green beans. Lipoxygenase is widely distributed in vegetables and evidence is mounting to support its involvement in off-flavour development and colour loss (Barrett and Theerakulkait, 1995). In order to optimize the blanching process of vegetables, it is essential to establish the kinetic model of the inactivation of the indicator enzyme. Isothermal and dynamic thermal approaches have been used to determine kinetic parameters (Lenz and Lund, 1980). In unsteady-state procedures the inactivation reaction occurs at a variable temperature and the data required are the concentration of the degraded enzyme and the temperature profile of the sample during the heating-cooling process (Rodrigo et al., 1998). The unsteady-state procedure is more flexible and can be applied to uniform and non-uniform heating situations; besides, a food medium, rather than an aqueous buffer solution, is always used for determining kinetic parameters (Welt et al., 1997). Svensson and Ericksson (1974b) studied the thermal inactivation of lipoxygenase in peas, but using kinetic parameters previously determined in pea press juice (Svensson and Ericksson, 1974a). Luna et al. (1986) studied the thermal destruction of peroxidase in the blanching of corn-on-the cob, considering the corn cob as a finite homogeneous cylinder. There is no information about kinetic parameters of thermal inactivation of lipoxygenase in foods using the unsteady-state procedure.
The objective of this work was to determine the kinetic parameters for thermal inactivation of lipoxygenase in cut green beans using unsteady-state methods.
II. MATERIALS AND METHODS
Green beans: green beans, variety Blue Lake, were obtained from a field close to Santa Fe city (Argentina); harvest was controlled by a member of the working group; once in the laboratory green beans were sized (diameter between 8.0 and 9.5 mm) and cut to a length of 20 mm. From each lot to be thermally treated a sample was obtained to determine green bean density. Chemical composition of green beans was experimentally determined.
Thermal treatment: For thermal treatment of cut green beans a stainless steel bath with a capacity of 40 dm3 was used. Bath temperature was measured with an Ellab type T probe connected to a Leeds and Northrup potentiometer; water bath was continuously agitated with a circulating pump. Cut green beans (approximately 40 g, corresponding to 36-41 pieces), with initial temperatures between 22.1 and 27.8ºC, were placed in a cylindrical basket made of stainless steel mesh (Nº 8), which was immersed in the water bath, rotating as a consequence of water agitation. Water bath temperatures used were 76, 82, 88 and 94 ºC, and the heating times ranged between 10 and 20 seconds. No modification of water bath temperature was detected when the green beans were immersed in it. Once the thermal treatment finished, and through a quick opening device of the cylindrical basket, green beans instantaneously fell down into a liquid nitrogen bath, where they were frozen in a very short time. Then the lot was packed in a pouch of high density polyethylene and stored at –20ºC. The analysis of lipoxigenase activity were performed along the week. For each time-temperature combination, a sample of fresh cut green beans was also frozen with liquid nitrogen, and used to determine initial lipoxygenase activity.
Experimental determination of convective heat transfer coefficient (h,W m-2 ºC-1) between water and cut green beans surface: To determine h, approximately 40 g of cut green beans and an acrylic cylinder ( D = 10 mm, L = 20 mm) were placed in the cylindrical basket. In the centre of the acrylic cylinder a type K thermocouple was inserted, connecting it to a Leeds and Northrup potentiometer. The cylindrical basket was immersed in the water bath and the experimental temperature profile of the acrylic cylinder centre was obtained ( ten tests were performed); bath temperatures ranged between 64 and 95ºC and the heating times between 0 and 240 seconds, measuring the temperature at intervals of 10 seconds; water bath temperature was also measured using an Ellab type T probe connected to a Leeds and Northrup recorder. Experimental centre temperatures were compared to theoretical temperatures varying the convective heat transfer coefficient between 200 and 1,500 W/m2 ºC, and determining the value of h that minimized the variance. Theoretical centre temperatures were obtained using the analytical solution of the heat conduction problem for a finite cylinder, with boundary condition of the third kind (Luikov, 1968) using 36 terms; acrylic thermal conductivity and thermal diffusivity used were 0.208 W/m ºC and 1.201 x 10-7 m2/s respectively (Califano, 1981). The experimental heat transfer coefficients determined were used to obtain the following Eqn. of variation of h with water bath temperature (T) and rotational speed of the cylindrical basket (N) (r2 = 0.9999):
h2 = 2.9508x106-8.8602x108 ln (TN)/TN (1)
Green bean thermal conductivity estimation: The equation of Maxwell-Eucken was used to estimate the thermal conductivity of green beans l (Wm-1 ºC-1) (Calvelo, 1984). The estimated values for green beans thermal conductivities as a function of green bean temperature were fitted with this Eqn. (r2 = 0.9975):
l = 0,4318 + 0.05117 T0.312 (2)
Experimental determination of cut green bean thermal diffusivity, a (m2 s-1): The same methodology as described in a previous point for determining h values was followed; the difference was that now the thermocouple was inserted in the centre of a cut green bean. Experimental centre temperatures were compared to theroretical temperatures, varying the green bean thermal diffusivity between 1.30 and 1.70 x 10-7 m2/s, and determining the value of a that minimized the variance. BiotR = hR/l and BiotZ = hZ/l were calculated using the values of h obtained from Eqn. (1), and green bean thermal conductivities from Eqn. (2) at a mean green bean temperature between T0 and Ta , initial and medium temperatures respectively ; analytical solutions used were the same as described previously. The following expression was obtained for the variation of a with water bath temperature (r2 = 0.9999):
a 0.5 = 3.65x10-4 + 3.386x10-7 T (3)
Experimental determination of lipoxygenase activity: Surrey (1964) method modified by the working group was used. A sample of 4-5 g was used for lipoxygenase activity determination in frozen fresh green beans, meanwhile 34-40 g were used for thermal treated frozen cut green beans. Retentions ( %) were calculated as (lipoxygenase activity of thermal treated sample/ lipoxygenase activity of fresh sample) x 100. For each thermal treatment performed a value of the lipoxygenase activity of the fresh sample was obtained.
Kinetic models:Two unsteady-state procedures were used to estimate kinetic parameters of lipoxygenase inactivation : A) the trial and error procedure developed by Lenz and Lund (1977a, 1977b, 1980) and applied by Luna et al.(1986); and B) the unsteady method used by Rodrigo et al. (1998). By procedure (A) a set of kinetic data (rate constant vs temperature) must be assumed; using this model an average enzyme activity retention, xaverage , in the whole volume (V) of the cut green bean is calculated as:
| (xaverage)calc. = | (4) |
where x is the enzyme activity retention in a point. This integral was evaluated by using the Gauss numerical integration, with 18 points; each of these points was calculated by using a first order inactivation reaction of the labile lipoxygenase, as
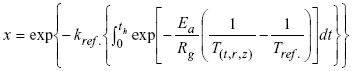 | (5) |
where T(t,r,z) was obtained from the solution of the unsteady heat conduction in a finite cylinder; the boundary conditions used for the heating step were of the third kind and cooling was considered to have a negligible effect on lipoxygenase inactivation; kref. was the reaction rate constant at the reference temperature, Tref was the reference temperature, Ea the activation energy and Rg was the universal gas constant.
By using procedure (B) the rate constant at reference temperature (mean value of the four temperatures used) and Ea were estimated by minimizing the following expression
| [(xaverage)exp. - (xaverage)calc.]2 | (6) |
by non linear regression; (xaverage)calc. were estimated as was described in the procedure (A). To statistically evaluate experimental error, minimization of Eqn. (6) was repeated by using as experimental data the twelve subsets generated by considering each time only eleven of the twelve blanching experiments. These calculations allowed to estimate the standard deviations for k85ºC and Ea.
Computer programms were developed in Lotus 123 version 5 (1994) in an IBM Aptiva PC. For some applications Table Curve software was used.
III. RESULTS AND DISCUSSION
Average chemical composition of green beans was: water = 91.75%; carbohydrates = 5.99% (by difference); proteins = 1.53%; lipids = 0.22%; ashes = 0.51%; green beans densities ranged from 902 to 988 kg/m3; meanwhile calculated equivalent diameters ranged from 8.26 to 9.21 mm.
Very good agreement was found between experimental and theoretical temperature profiles, indicating that the model predicted the temperature inside the cut green bean very well; heat up period represented all the process time, justifying the use of the unsteady procedure.
Table 1 shows the experimental retention (%) of cut green bean lipoxygenase activity as a function of different combinations of time and temperature; letters (a), (b) and (c) show the order in which the combinations were used to make iterations of procedure (A).
In order to determine the kinetic parameters of lipoxygenase inactivation by procedure (A) it was necessary to assume starting values for k85ºC and Ea ; no information was available for green beans, so a set of values for lipoxygenase inactivation in pea press juice, k85ºC = 5.12 s-1 and Ea = 479 KJ/mol, was chosen (Svensson and Ericksson, 1974a). Preliminary testing using these values allowed to find the best set of definitive starting values, k85ºC = 148 s-1 and Ea = 154.85 KJ/mol.
Table 1. Experimental lipoxygenase retention as function of time-temperature heat treatment.

(a), (b) and (c) are subsets used for iterations in procedure A.
For subset (a) the number of iterations were five, meanwhile for subsets (b) and (c) three ones were necessary. Table 2 shows the values of kref. at four temperatures and the corresponding Ea obtained. The average of three values (between parenthesis is the standard deviation) were the following: k76ºC = 27.19 (9.44) s-1; k82ºC = 92.95 (7.46) s-1; k88ºC = 212.10 (52.73) s-1; k94ºC = 407.79 (56.68) s-1; Ea = 160.57 (8.07) KJ/mol.
Table 2. Kinetic parameters of cut green bean lipoxygenase inactivation determinedby procedure A.

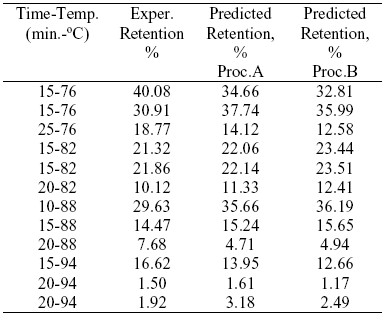
The kref. and Ea that minimized Eqn. (6) using procedure B were the following: k85ºC = 150 s-1 and Ea = 164 KJ/mol; standard deviations obtained were 26.28 s-1 and 4.72 KJ/mol respectively. The residual sum of squares (RSQ) between experimental retention values and those predicted by using rate constants and Ea obtained by procedure (A) was 154.24, meanwhile when k85ºC and Ea obtained by procedure (B) were used the RSQ was 197.33. Average Ea and rate constants obtained by procedure (A) predicted lipoxygenase retentions values closer to experimental ones (Table 3).
Table 3. Experimental and theoretical lipoxygenase retentions as function of time-temperature heat treatment.
III. CONCLUSIONS
Both unsteady-state methods which were tested to determine kinetic parameters of green bean lipoxygenase inactivation during blanching treatment gave similar results. Predicted and observed lipoxygenase retentions showed good agreement. The Ea and k values obtained proved that green bean lipoxygenase might be easily inactivated at temperatures normally used in a blanching process. The kinetic parameters that were determined and the appropriate mathematical model could be used to optimize and improve thermal treatment of green beans using lipoxygenase as index of blanching adequacy.
Acknowledgements
The authors acknowledge the financial support of Universidad Nacional del Litoral (Programación CAID) and the technical assistance of Adriana Avalle.
REFERENCES
Barrett, D.M. and Theerakulkait, C. Quality indicators in blanched, frozen, stored vegetables. Food Technology, 49, 62-65 (1995). [ Links ]
Califano, A.N. Transferencia de calor y materia durante el escaldado de papas. PhD thesis. Universidad Nacional de La Plata. Argentina (1981). [ Links ]
Calvelo, A. Modelos para cálculo del tiempo de congelación de alimentos. In: La Refrigeración Como Medio Para Disminuir las Pérdidas Post-Cosecha, vol. I. Pp.67-98. Edited and published by Secretaría de Ciencia y Técnica. Buenos Aires, Argentina (1984). [ Links ]
Lenz, M.K. and Lund, D.B. The Lethality-Fourier number method: Experimental verification of a model for calculating temperature profiles and lethality in conduction-heating canned foods. Journal of Food Science, 42, 989-996, 1001 (1977a). [ Links ]
Lenz, M.K. and Lund, D.B. The Lethality-Fourier number method: Experimental verification of a model for calculating average quality factor retention in conduction-heating canned foods. Journal of Food Science, 42, 997-1001 (1977b). [ Links ]
Lenz, M.K. and Lund, D.B. Experimental procedures for determining destruction kinetics of food components. Food Technology, 34, 51-55 (1980). [ Links ]
Luikov, A. Analytical heat diffusion theory. Pp. 283-286. New York: Academic Press (1968). [ Links ]
Luna, J.A., Garrote, R.L. and Bressan, J.A. Thermo-kinetic modeling of peroxidase inactivation during blanching-cooling of corn on the cob. Journal of Food Science, 51, 141-145 (1986). [ Links ]
Rodrigo, C., Mateu, A., Alvarruiz, A., Chinesta, F. and Rodrigo, M. Kinetic parameters for thermal degradation of green asparagus texture by unsteady-state method. Journal of Food Science, 63, 126-129 (1998). [ Links ]
Surrey, K. Spectrophotometric method for determination of lipoxidase activity. Plant Physiology, 39, 65-70 (1964). [ Links ]
Svensson, S.G. and Ericksson, C.E. Thermal inactivation of lipoxygenase from peas (Pisum sativum L.) III. Activation energy obtained from single heat treatment experiments. Lebensmittel-Wissenschaft und-Technologie, 7, 142-144 (1974a). [ Links ]
Svensson, S.G. and Eriksson, C.E. Thermal inactivation of lipoxygenase from peas (Pisum sativum L.) IV. Inactivation in whole peas. Lebensmittel-Wissenschaft und-Technologie, 7, 145-151 (1974b). [ Links ]
Welt, B.A., Teixeira, A.A., Balaban, M.O., Smerage, G.H., Hintinlang, D.E. and Smittle, B.J. Kinetic parameter estimation in conduction heating foods subjected to dynamic thermal treatments. Journal of Food Science, 62, 529-534, 538 (1997). [ Links ]
Williams, D.C., Lim, M.H., Chen, A.O., Pangborn, R.M. and Whitaker, J.R. Blanching of vegetables for freezing-Which indicator enzyme to choose. Food Technology, 40, 130-140 (1986). [ Links ]
Received: September 16, 2001.
Accepted for publication: August 28, 2002.
Recommended by Guest Editors J. Cerdá, S. Díaz and A. Bandoni.














