Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Latin American applied research
versión impresa ISSN 0327-0793
Lat. Am. appl. res. v.33 n.2 Bahía Blanca abr./jun. 2003
Kinetic model of pectin demethylation
D. Constenla and J.E. Lozano
Planta Piloto de Ingeniería Química (UNS-CONICET)
Camino "La Carrindanga" km 7. CC 717. (8000) Bahía Blanca. ARGENTINA.
{dconstenla, jlozano}@plapiqui.edu.ar
Abstract- Pectins are polysaccharides that act as a cellular binder in the peel of many different fruits and vegetables. An important feature of pectins is the esterification of the galacturonic acid residues with methanol. The degree of methylation is defined as the number of moles of methanol per 100 moles of galacturonic acid. The objective of the present work was to study the acid demethylation of apple pectin at different temperatures, by following changes in the degree of methylation. A solution of high methoxyl apple pectin was demethylated with concentrated HCl (pH = 0.5). The processes were carried out for 120 min at T = 80°C and 400 min at T = 65°C and T = 50°C. Anhydrogalacturonic acid content, degree of methylation and intrinsic viscosity as a function of reaction time were determined. Results showed pectin demethylation followed a second order kinetics and demethylation rate increased exponentially with temperature. Results also showed pectin "purity", as anhydrogalacturonic acid content, increased with demethylation. However, intrinsic viscosity reduced with reaction time. This behavior was associated with the hydrolysis of non-polyuronic material during the acid treatment, lowering in that way the average molecular weight.
Keywords- Pectin. Demethylation. Kinetic Model. Bio-Polymer. Low Methoxyl.
I. INTRODUCTION
Pectic substances are glycosidic macromolecules exclusively present in plants. They are industrially extracted to be used as a food additive. The pectic substances act as lubricating or cementing agents in the cell walls of plants, also they are nutritional fibers and have many interesting medical properties (Kravtchenko et al., 1992).
Pectins are complex polysaccharides, basically polymers of a -D-(1-4)- linked galacturonic acid, but they also contain neutral sugars such as L-rhamnose, D-galactose, L-arabinose, D-xylose, etc. (Schols and Voragen, 1996). As they occur naturally, the galacturonic acid sugars are usually highly esterified and are interrupted by L-rhamnose residues.
Occasionally, at points along the backbone there are sites of extensive branching involving neutral sugars, these structural features giving rise to terminology "smooth" and "hairy" regions when referring to regions of the pectin backbone (Morris et al., 2000). Depending upon sources, galacturonic acid residues of the pectin are esterified to various degrees, and the size and composition of neutral sugar side chains vary.
Pectins have been divided into two groups in the market: those containing more than 50% esterification (high methoxyl pectin, HMP) and those containing less than 50% esterification (low-methoxyl pectin, LMP). The main sources of HMP are citrus peel and apple pomace. Commercial LMP are obtained from HMP by chemical demethylation.
Pectins provide beverage viscosity, LMP may be used as a gelling agent in low-sugar products, such as low-calorie jams and jellies, confectionery jelly products, and other food applications. The heat reversibility of LMP gels can be utilized in bakery jams and jellies for glazing, microwaving, baking, sterilizing or pasteurizing, and freezing/thawing (Kertesz, 1951).
The anhydrogalacturonic acid content (AGA), degree of methylation (DM), molecular size, distribution of carboxyl groups, and therefore the charge on a pectin molecule are important to the functional properties of pectin solutions and can affect structural and textural properties of pectin gels. Pectin viscosity is also affected by extrinsic factors such as temperature, concentration of solute, pH, and the presence of salts and co-solutes.
Although some LMP occurs in plants, they are usually manufactured from HMP. There are four methods of demethylation according to the agents used: acids, alkalis, enzymes and ammonia in alcohol. Acid demethylation is commonly used to manufacture LMP. Depolymerization is the main disadvantage of the acid treatment which hydrolyzes glycosidic bonds (Kertesz, 1951). Kim et al. (1978) showed that using higher concentration of acid at low temperatures gave less depolymerization during demethylation than when lower concentration and higher temperatures were used.
Padival et al. (1979) stated that demethylation of pectin extract at 80 and 90 °C yielded LMP of poor gelling characteristic and molecular weight decreased with increased temperature of demethylation.
The objectives of this study were (i) to prepare LMP by acid demethylation of apple HMP; (ii) to determine kinetics of demethylation at different temperatures; and finally (iii) to characterize the obtained product.
II. MATERIAL AND METHODS
High methoxyl apple pectin obtained in the laboratory (AGA = 60%, DM = 80%) was used to prepare LMP. HMP was obtained from dried pomace by acid extraction. Filtration, concentration and alcoholic precipitation follow the extraction step. The pectin was dried, milled and characterized (Constenla et al., 2002).
Pectin dispersions in distilled water were prepared at a concentration of 30 mg/g, under agitation at room temperature. The pH was adjusted to 0.5 at work temperature (Orion pHmeter with high temperature electrode, Orion Research Inc., Beverly, USA) by HCl concentrated addition.
Acid demethylation at 50, 65 and 80°C were carried out in a thermostatized bath with constant agitation. The pH did not change during reaction. To measure the degree of demethylation 25 mL of sample being de-esterified were pipetted out at different intervals depending on temperature (e.g. 60 min in the range 50 - 65°C and 15 min for 80°C), cooled, and the pectin precipitated with 96 °GL ethanol. The precipitate was washed 3-4 times with 70 °GL ethanol to remove acid excess, then filtered through Whatman N°4 filter paper and dried under vacuum at 40 °C. Samples were stored at room temperature.
A. Pectin Characterization
AGA content was quantitatively determined by the colorimetric phenyl-phenol method (Blumenkrantz and Asboe-Hansen, 1973) with an UV / VIS Perkin – Elmer Lambda 3 Spectrophotometer (Illinois, USA), at 520 nm in a 1cm quartz cell.
DM was determined by gas chromatography (Lee et al., 1975). A stock solution of pectin was prepared at a concentration of 8.33 mg/g in distilled water. Pectin methyl esters were hydrolyzed as follows: 1 mL of NaOH 1 N was added to 12 mL pectin solution. This solution remained for 30 min. at room temperature, and finally was diluted with distilled water to 20 g. Aliquots of the hydrolyzed pectin samples were analyzed for methanol. A Varian 3700 gas chromatograph (Walnut Creek,Ca, USA) equipped with a stainless steel filled column (Porapak N 80/100 mesh) of 2 m x 2 mm i.d. was used. The oven temperature was fixed to 115ºC and the injector and detector (FID) temperatures were 210ºC and 220ºC, respectively. The carrier gas (N2) flow rate and sample volumes were 20 mL/min and 2 m L, respectively. Standard curve was prepared with known amounts of methanol in distilled water. Fitting the calibration curve resulted in a straight line through the origin with a regression coefficient r2 = 0.9975. DM was calculated as the ratio molar of methanol to AGA, expressed as a percentage.
Apple pectin molecular weight was estimated by applying the Mark Houwink equation, relating the intrinsic viscosity, [h ], with viscosity-average molecular weight, Mv:
![]() ,(1)
,(1)
where k and a are constants. Both k and a depend on temperature, solute and solvent characteristics. A large number of models have been used to deduce [h ]-Mv relationships (da Silva and Rao, 1992). In the case of pectin solution in sodium phosphate buffer (0.1 M; pH=7) may be assumed to be k = 0.3 mL/g and a = 0.613 (Arslan, 1995).
Equations relating relative viscosity (solution to solvent) h r with [h ] and pectin concentration C are:
| , (2) |
| , (3) |
where kH and kK are the Huggins and Kraemer coefficients respectively.
Intrinsic viscosity [h ] can be determined by the combined extrapolation at C=0 of Huggins (Eqn.2) and Kraemers (Eqn.3) equations. The expressions (h r – 1)/C and ln (h r)/C are known as viscosity index and logarithmic viscosity index, respectively.
Determination was made in the range of pectin concentration 1 – 4 mg/g. Viscosity measurements were performed at 25 ±0.1ºC with a Bohlin CVO stress controlled rheometer (Glousestershire, England) using concentric cylinders geometry (C25). Constant shear stress (s = 0.1Pa) was selected.
III. Results and Discussion
The changes of DM with time at different temperatures are shown in Fig. 1. Many workers describing acid demethylation use a first order reaction. It was observed, in the present work, that a second order kinetics was more appropriate.
| , (4) |
where DM0 is the initial degree of methylation and DM is the degree of methylation at time t. The K values represent the rate constants of demethylation in min-1.
Table 1 lists reaction rate constants at 50, 65 and 80°C. While K values were very similar for treatments at 50 and 65°C, a higher reaction rate was observed at 80°C.
Figure 2 shows the variation of AGA content during demethylation. Results indicated that AGA content increases in the beginning of reaction to reach a constant value. This may be attributed to simultaneous hydrolysis of non-uronic material. Kertesz (1951) also reported similar results.
Depending on the plant source, raw material quality, extraction method and chemical treatment after extraction, pectin of variable average molecular weight can be obtained. Generally a high molecular mass is more favorable for gel formation.

Figure 1. Degree of methylation as a function of reaction time.
Table 1. Reaction rate constants of pectin demethylation at pH = 0.5 (Eqn. 4).


Figure 2. Changes in AGA content during acid demethylation of pectin at 50, 65 and 80°C.
The intrinsic viscosity was determined by extrapolation of Eqns. 2 and 3. Both plots are shown in Fig.3 for a pectin sample obtained after 80 min of demethylation at T = 80°C.
Intrinsic viscosity is a characteristic of macromolecules that is related directly to their ability to disturb flow and indirectly to their size and shape (Tanglertpaibul and Rao, 1987). In very dilute solutions, the effects of overlapping and interaction of macromolecules are irrelevant (Chou and Kokini, 1987). In these case the second terms of Eqns. (2) and (3) are negligible and the equations will be linear. At high concentrations and molecular size the second term comes into play and a nonlinear equations results.
The transition from diluted to concentrated solution in this case occur at h r = 1.5. This is consistent with observations of da Silva et al. (1992).
The viscosity-average molecular weight was calculated with Eqn. 1, using the mean value of the intrinsic viscosity calculated in Fig. 3. Values obtained with Huggins (kH = 0.439) and the Kraemers (kK = -0.086) coefficients, were consistent with random coil molecules, and similar to previous values reported by Souza and Andrade (1999) for amidated LMP and by Chou and Kokini (1987) for apple pectin. Values of kH ranging from 0.37 to 0.59 were reported previously for non-amidated pectins with different degrees of esterification (Souza and Andrade, 1999).
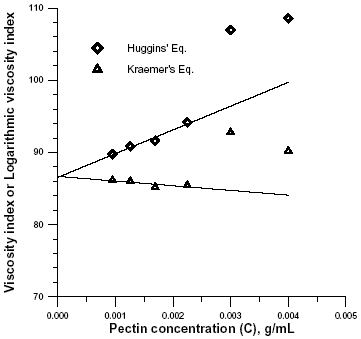
Figure 3. Determination of intrinsic viscosity of pectin by combined extrapolation in 0.1 M sodium phosphate, pH =7, at 25°C.
Figure 4 shows the decrease in viscosity-average molecular weight during demethylation at 80°C. It was determined that Mv remained practically constant at 60000 when the pectin was treated at lower temperatures (50 and 65°C). Kim et al. (1978) also found that pectin demethylation at lower temperatures caused lower average molecular weight reduction.
It was observed that Mv decreased as demethylation progressed (Fig. 5). This reduction was very important to be attributed only to demethylation, with certainty hydrolysis of poligalacturonic acid linkage to be produced. Otherwise during demethylation at low temperature, the ester content was reduced to different levels with a minimum effect on polymer size.
These results are indicating that higher rates of demethylation imply a reduction of pectin molecular mass, affecting the gel formation ability.

Figure 4. Changes in viscosity-average molecular weight during demethylation at T = 80°C.

Figure 5. Viscosity-average molecular weight as a function of degree of methylation at 80°C.
IV. Conclusions
Temperature affects the rate of demethylation and the product characteristics. The rate of demethylation increased with temperature. Demethylation reaction under the present experimental conditions (initial concentration of pectin, pH and temperatures) showed a second order kinetics. An increase in AGA content with temperature was also observed during acid demethylation. Acid demethylation using HCl (pH = 0.5) at higher temperatures resulted in partial pectin depolymerization. Low methoxyl pectins produced by demethylation at temperatures higher than 65°C resulted in the break down of pectin to smaller molecular species. Average molecular weights were unaffected at the lower temperatures assayed.
Acknowledgments
This work was supported by research grants from CONICET (Consejo Nacional de Investigaciones Científicas y Tecnológicas) and Universidad Nacional del Sur, Argentina.
REFERENCES
Arslan, N., "Extraction of Pectin from Sugar-Beet Pulp and Intrinsic Viscosity-Molecular Weight Relationship of Pectin Solutions", J. Food Sci. Technol. 32(5), 381-385 (1995). [ Links ]
Blumenkrantz, N. and G. Asboe-Hansen, "New Method for Quantitative Determination of Uronic Acids", Analytical Biochemistry 54, 484-489 (1973). [ Links ]
Constenla, D., A.G. Ponce and J.E. Lozano, "Effect of Pomace Drying on Apple Pectin", Lebensmittel - Wissenschaft und – Technol. 35(3), 216-221 (2002). [ Links ]
Chou, T.D. and J.L. Kokini, "Rheological Properties and Conformation of Tomato Paste Pectins, Citrus and Apple Pectins", J. Food Science 52(6), 1658-1664 (1987). [ Links ]
da Silva, J.A.L. and M.A. Rao, "Viscoelastic Properties of Food Hydrocolloid Dispersions" In Viscoelastic Properties of Food. M.A. Rao and J.F. Steffe (De.) Elsevier Applied Science, New York. 371-434 (1992). [ Links ]
da Silva, J.A.L, M.P. Gonçalves and M.A. Rao, "Rheological Properties of High-Methoxyl Pectin and Locust Bean Gum Solutions in Steady Shear", J. Food Science, 57(2), 443-448 (1992). [ Links ]
Kertesz, Z.I., "The Pectic Substances", Interscience Publishers, Inc. New York (1951). [ Links ]
Kim, W.J., C.J.B. Smit and V.N.M. Rao, "Demethylation of Pectin Using Acid and Ammonia", J. Food Science 43, 74-78 (1978). [ Links ]
Kravtchenko, T.P., A.G.J. Voragen and W. Pilnik, "Analytical Comparison of Three Industrial Pectin Preparations", Carbohydrate Polymers 18, 17-25 (1992). [ Links ]
Lee, C.Y., T.E. Acree and R.M. Butts, "Determination of Methyl Alcohol in Wine by Gas Chromatography", Analytical Chemistry 47(4), 747-748 (1975). [ Links ]
Morris, G.A., T.J. Foster and S.E. Harding, "The Effect of the Degree of Esterification on the Hydrodynamic Properties of Citrus Pectin", Food Hydrocolloids 14, 227-235 (2000). [ Links ]
Padival, R.A., S. Ranganna and S.P. Manjrekar, "Low Methoxyl Pectins from Lime Peel", J. Fd Technol. 14, 333-342 (1979). [ Links ]
Schols, H.A. and A.G.J. Voragen, "Complex Pectins: Structure Elucidation Using Enzymes", Pectins and Pectinases. J. Visser and A.G.J. Voragen Ed. Elsevier Science B. V. (1996). [ Links ]
Souza, R.C.R. and C.T. Andrade, "Gel Formation in Amidated Low Methoxyl Pectin Systems at Unusually Acidic Conditions", Latin American Applied Research 29, 145-149 (1999). [ Links ]
Tanglertpaibul, T. and M.A. Rao, "Intrinsic Viscosity of Tomato Serum as Affected by Method of Determination and Methods of Processing Concentrates", J. Food Science 52(6), 1642-1645 (1987). [ Links ]
Received: September 16, 2001.
Accepted for publication: August 28, 2002.
Recommended by Guest Editors J. Cerdá, S. Díaz and A. Bandoni.














