Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Latin American applied research
Print version ISSN 0327-0793
Lat. Am. appl. res. vol.33 no.2 Bahía Blanca Apr./June 2003
Low resolution 1H-Pulsed NMR for sugar cristallization studies
A. Gallo1, M. F. Mazzobre2, M. P. Buera2 and M. L. Herrera2
1 Departamento de Tecnología, Universidad Nacional de Luján, 6700 Buenos Aires, Argentina
agallo@unlu.edu.ar
2 Departamento de Industrias, Universidad de Buenos Aires,1428 Buenos Aires, Argentina.
lidia@di.fcen.uba.ar
Abstract—The stability of food and other labile biological systems containing sugars has been related to the amorphous characteristics of the matrices which they formed, being inversely related to the degree of crystallization. It is thus necessary to explore the applicability of simple, non-destructive and reliable methods to analyze sugar crystallization. Solid fat content is a well-stablished AOCS method to study solid content in lipid systems. However, there are no literature reports on the use of this method to analyze sugar crystallization. Crystallization kinetics of concentrated trehalose and trehalose/salts solutions was followed by proton pulse nuclear magnetic resonance (1Hp -NMR). Three different concen-trations (XT= 0.60, 0.63 and 0.66) of trehalose solutions were crystallized to 25, 20, 15, 10 and 5°C and the degree of crystallization was investigated by following the index of solids with time. Crystallization rate was determined by a combined effect of supercooling and molecular diffusion. By analyzing trehalose systems by 1H p-NMR, it was possible to confirm the effect of divalent cations on retarding sugar crystallization.
Keywords— NMR. Crystallization. Trehalose Solutions. Divalent Cations.
I. INTRODUCTION
Sugars have frequently been used in food and pharmaceutical fields for protecting both proteins and cells during freezing and drying due to their ability to form glasses, to mimic the hydrogen bonding character of water, to increase the surface tension of the bulk solvent, to prevent thermotropic phase separations in lipid bilayers, and to prevent the fusion of membranes (Conrad and de Pablo, 1999). Trehalose is a nonreducing disaccharide of glucose. It occurs naturally in many organisms which suffer dehydration stresses. Trehalose´s success as a cryoprotectant has been attributed to its strong interaction with water and lipid membranes, its unique chemical stability, and its glass-forming behavior when amorphous (Crowe et al., 1996). The stabilizing effect of sugars has been inversely related to the degree of crystallization (Suzuki et al., 1997; Cardona et al., 1997). Divalent cations were reported to delay crystallization kinetics of sugars (Mazzobre and Buera, 1999).
The crystallization of sugars is also important in the food industry as evidenced by the many processes where the degree of crystallinity of sugars is critical to acceptance of the final product, i.e., storage stability and quality of milk powders are significantly affected by the physical state of lactose, one of the main components in regular milk powders (Jouppila and Roos, 1994). Crystallization of lactose in ice-cream, condensed milk and milk powders is considered undesirable, while in products such as milk chocolate lactose crystallization is desirable. Likewise sucrose crystallization evidenced by graining in boiled sweets is considered to be a defect whereas the fine crystals present in fondant icing are desirable because they help enable the icing to retain its shape in confectionery products (Hartel and Shastry, 1991; Hartel, 1992).
The control of lactose and sucrose crystallization during processing is important for a wide variety of products and there is a need for a suitable method to determine the degree of crystallization which would occur under specific processing conditions (Kedward et al., 1998). Nuclear Magnetic Resonance (NMR) is a noninvasive technique often used for the examination of biological systems. This technique allows the determination of relaxation effects of protons containing in different states. Low resolution 1H- pNMR is widely employed to determine the solid/liquid ratio in fats (AOCS official method). However, no previous references have been found for the application of this technique to determine the degree of crystallization in sugar solutions. The most common techniques in sugar studies are the evaluation of spin-spin and spin-lattice relaxation times. The experiments involve the study of a one phase systems and are usually performed in dehydrated systems (Cornillon, 2000) or vitreous systems (Rubin et al., 1990). Diffusion of sugars in aqueous solutions was also investigated performing spin echo and spin-spin relaxation experiments (Martin et al., 1999) and the determination of soluble solids content in fruits (Cho et al., 1993; Keener et al., 1997) by NMR was also reported.
The aim of the present work was to analyze the applicability of low resolution proton pulse nuclear magnetic resonance (1H p-NMR) to describe crystallization behavior of sugars. Isothermal crystallization behaviour of trehalose solutions with time was analyzed and the effect of salts of divalent cations such as ZnCl2.4H2O, CaCl2.2H2O and MgCl2.6H2O on crystallization kinetics was also investigated.
II. METHODS
A. Sample preparation
a ,a -Trehalose dihydrate, (a -D-Glucopyranosyl-1-1-a -D-glucopyranoside) from Saccharomyces cerevisiae, 99% was used without any further purification. HPLC water was used for all experimental work. CaCl2.2H2O, ZnCl2.4H2O and MgCl2.6H2O were analytical grade. Concentrated trehalose solutions (mass fraction of sugar (XT) of 0.60, 0.63 and 0.66) were prepared by weighting carefully cold water and trehalose. Salts were added to the XT= 0.60 solution in a molar ratio trehalose/salt 5:1. The solutions were heated at 70°C with stirring for 1 h, then the NMR tubes were filled with the samples and placed into a bath at crystallization temperature. Selected crystallization temperatures were 5, 10, 15, 20 and 25°C.
B. Measurement of solid content
Solids contents of the samples were measured by 1H pulsed nuclear magnetic resonance (1H-pNMR) in a Minispec PC/120 series NMR analyzer (Bruker, Karlsruhe, Germany). A 20 MHz pulse of radio frequency radiation, lasting only 2 microseconds at a 90° between incident pulse and magnetic field vector (AOCS official method), was used. This pulse excited the hydrogen nuclei in all phases, and when the pulse was switched off, the nuclei returned to their original state, emitting an NMR signal as they did so. The initial amplitude of the signal is proportional to the total number of hydrogen nuclei in the sample. The signals due to nuclei in different physical phases decay at different rates. The number of hydrogen nuclei in the liquid phase and the total number of hydrogen nuclei in liquid and solid were measured. The solids fraction is given by the following equation:
 | (1) |
where SA1 is the signal amplitude proportional to the total number of hydrogen nuclei, SA2 is the signal amplitude proportional to the hydrogen nuclei in the liquid phase, and F is a factor to correct the dead-time of the receiver since it is not possible to measure the samples at time zero. F was previously determined by using certified standards of plastic in oil where the actual solid content is known exactly. The relaxation properties of standards and any other systems such as fats, polymers and food emulsions follow proportionality. F factor of proportionality can be determined by measuring the solid fraction by the indirect method Cd 16-81.
 | (2) |
The ratio between this value and the one obtained by the direct method Cd 16b-93 is the F factor. Different F factors can be obtained for different fats, different polymorphic forms, emulsions and polymers. In the polymer industry, the solid ratio experiments becomes very important since it is possible to measure the degree of polymerization in actual chemical processes by measuring the increasing amount of solid in the reaction as it proceeds. With the same methodology, F factors can be determined for sugars. The F' factor can also be determined by comparing the NMR direct method solid content value to the solid content value obtained by other method such as DSC. Trehalose is a particularly simple case because it has just one crystalline form when it crystallizes from water at the conditions selected for this study. The F factor for trehalose was 1.05. It is valid to assume that the hydrogen nuclei contents and relaxation properties of the trehalose and the standards are similar. Samples were run in quadruplicate and the values were averaged. Solids fraction was measured as a function of time.
C. Differential Scanning Calorimetry (DSC).
The thermal transitions of the trehalose systems with and without MgCl2 were measured by differential scanning calorimetry (DSC) (Mettler DSC30, TA4000, graphware TA72). Hermetic aluminum pans were employed, an empty aluminum pan was used as reference, and the samples were scanned at a heating rate of 10°C/min. The instrument was calibrated with indium and Tg was calculated as the onset temperature of the transition.
D. Light polarized microscopy
To check the behavior found by NMR, crystallization was observed by polarized light microscopy. A Leitz microscope model Ortholux II (Leitz Co., Wetzlar, Germany) with a controlled temperature platform was used to follow crystallization. In the crystallization tests, for microscopic observation the samples were melted with stirring at 70°C and held at this temperature for 1 h. Samples were then placed on a concave slide at the selected crystallization temperature and covered with a cover slip. The platform temperature was controlled by a Lauda TUK cryostat (Werklauda, Königshofen, Germany), which was filled with a mixture of ethylene glycol/water 3:1 (v/v). Photographs of the crystals were taken with a Leitz-Vario-Orthomat camera under polarized light during crystallization with time. Magnification was 25X. Scale for all photographs: 1cm=137.5 m m. The selected example to show the morphology was the trehalose solution of XT= 0.60 and this solution with the addition of salts crystallized on a microscope slide at 20°C. It is very difficult to quantify crystallization kinetics with microscopy at the sugar concentrations used in this study because many fields are empty, very different crystal sizes are present at the same time and they are sometimes aggregated. In addition, to obtain results with statistical significance, at least 200 crystals must be analyzed for their area. For these samples, more than 20 repetitions must be done to reach that crystal number. However, qualitative results can be obtained. Crystallization studies were also performed at 10 and 15°C.
III. RESULTS AND DISCUSSION
A. Crystallization of Trehalose Solutions
Table 1 shows the supersaturation (b ) and the difference between crystallization and glass transition temperatures (T-Tg) for the three trehalose solutions (XT= 0.60, 0.63, 0.66) at different temperatures. Supersaturation is defined as b =XT-XSL, where XSL is the amount of trehalose soluble at saturation. Solubility at different temperatures were reported by Miller et al., (1997). As evident from Table 1, supersaturation decreases when temperature increases. In addition, (T-Tg) values are an indication of the degree of plasticization of the sugar molecule by water. At low (T-Tg) values, although supersaturation is high, sugar molecule have low molecular mobility since water plasticization is poor.
Table 1: Characteristics of the systems at the conditions selected for the crystallization studies.
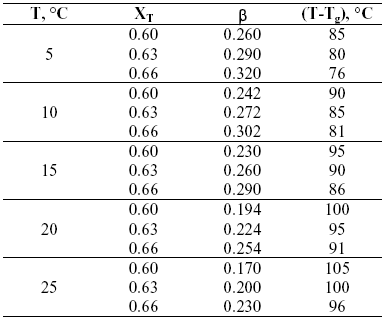
Figure 1 shows the solid or crystalline fraction (Xs) as a function of time for the trehalose solutions incubated at 25°C. The curves had sigmoidal shape, there was an induction time for sugar crystallization, followed by a period of increasing crystallization rate and a last period of decreasing crystallization rate. At 25°C trehalose crystallization was faster in concentrated solutions (XT= 0.63 and 0.66). Probably at this temperature no molecular diffusion limitations take place, being sugar crystallyzation mainly governed by supersaturation.

Figure 1. Change in the solid fraction (Xs) for solutions of initial mass fraction of trehalose (XT) 0. 60, 0.63 and 0.66 during storage at 25°C.

Figure 2. Effect of supersaturation and temperature on the maximum crystalline fraction (related to the super-saturation, b ) (a) and to the crystallization rate (b) of trehalose solutions at several temperatures.
Figure 2a, shows the maximum level of crystallized sugar (related to the supersaturation value) after 9 h of storage at the selected temperatures. The slope of the linear portion of the curves Xs vs. time (k) (as indicated in Figure 1 for 25°C) was calculated as an indication of the crystallization rate and plotted as a function of temperature (Figure 2b). As shown in Figure 2 (a,b), crystallization at 5°C did not occur after 9 h of storage, although b was high. Crystal growth drop off at high supersaturations or supercoolings due to the increased influence of mass transfer inhibition (Hartel 1992). As the driving force (b ) increases, the crystal growth rate is expected to increase. However, at extremely high driving forces (very low temperatures or very high concentration), crystal growth goes to zero due to inhibition of molecular mobility. Systems that do not undergo glass transition such as edible fats and oils show higher crystallization rates for higher termodynamic driving force, that is higher supercoolings. In the case of sugar solutions, although high supercooling values thermodynamically make the crystallization process favorable, they are kinetically inhibited due to the low molecular mobility in the solution phase. It was reported that sugar solutions showed this behavior (Hartel 1992) but it was not quantified previously by NMR. Both, the maximum level of crystallized sugar and the rate of crystallization (Figure 2 a,b) were higher for the intermediate concentration solution (XT= 0.63) and at temperatures between 10 and 20°C, diminishing with further increasing temperature. Solid fraction in the solution XT= 0.63 reached almost the maximum solid fraction expected (related to the supersaturation value) at 10°C (Figure 2a). However, for solutions XT= 0.60 and 0.66, the maximum solid fraction attain was lower than the expected on the basis of b. Although supersaturation is higher for the solution XT=0.66, it became very viscous and therefore no crystallization occurred at temperatures lower than 20°C due to molecular diffusion limitations.
B. Effect of salts on crystallization kinetics
The modification of crystallization kinetics because of the addition of salts to the XT= 0.60 trehalose solution is shown in Figure 3. As in all the systems the anion (Cl-) was kept constant, the differential behaviour observed for the salts could be attributed to the cations. Among the selected chlorides, CaCl2 was very efficient in delaying crystallization. After 6.5h solid fraction still remained lower than 0.01. MgCl2 efficiency was intermediate between CaCl2 and ZnCl2. The three salts diminished crystallization rate and the maximum solid fraction (Table 2). However, the curves showed a similar shape than the one exhibited by the trehalose solution alone. Divalent cations were reported to have a greater effect than monovalent cations on stabilizing proteins (Carpenter et al., 1987). It was expected that the effect of divalent cations on crystallization rate followed the order Mg2+ > Zn2+ > Ca2+ in agreement with their polarizing character. That is, the ratio charge/radius. CaCl2 showed an unexpected behavior that may be due to solvation of Ca2+ ions with trehalose molecules instead of water.
Low resolution 1H-NMR was presented as a suitable method to follow sugar crystallization degree along time, by determining the solid fraction. Parallel qualitatively experiments by two others methods, differential scanning calorimetry (DSC) and light polarized microscopy (LPM), confirmed the results obtained by 1H-p-NMR.

Figure 3 Change in solid fraction with time at different temperatures for the trehalose solution and with the addition of ZnCl2, MgCl2 and CaCl2 in molar ratios trehalose/salt 5:1.
Table 2. Maximum solid fraction (Xs) and rate of crystallization (k) for trehalose and trehalose-salt systems of total mass fraction of solids XT=0.60

Figure 4 shows the DSC thermograms obtained for trehalose and trehalose/MgCl2 solutions after storage at 25°C. The endotherms indicate the solubilization of sugar which crystallized during the previous storage. The area of the endothermal peaks are thus related to the amount of crystalline sugar formed. In solutions containing only trehalose, the sugar crystallized after 2h at 25°C (Figure 4a). However, the absence of the endothermal peak in the trehalose/MgCl2 thermogram solution up to 23hs of storage indicate that trehalose crystallization was delayed in presence of MgCl2.

Figure 4. DSC thermograms for supersaturated solutions of trehalose (a) or trehalose-MgCl2 (b) of mass fraction of solids (xT) 0.60 after 0, 2, 5 and 23 h at 10°C.
C. Confirmation of salts effect
Spot observations of trehalose and trehalose/salt solutions were done by LPM. Trehalose solutions of XT= 0.60 with and without salts were crystallized on a microscope slide at 10, 15 and 20°C. For the trehalose solution crystallized at 20°C, small crystals were observed after 1 h. Figure 5a shows the morphology of trehalose crystals after 2 h 30 min. Trehalose solution XT= 0.60 in presence of CaCl2 did not show crystals during the first 48 h.. In the presence of ZnCl2 and MgCl2 crystallization was not observed during the first 2 h. The crystals were smaller than those of trehalose alone and showed a different morphology as shown for trehalose/MgCl2 in Figure 5b. As observed in solid systems (Longinotti et al., 2001), salts constrain the dimensionality of sugar crystal growth. The obtained qualitative results were in agreement with the order of crystallization delay observed by NMR for the three crystallization temperatures.


Figure 5. Images of crystals obtained by light polarized microscopy after 2h 30 min at 20°C. a) trehalose solution (XT = 0.60), b) trehalose/MgCl2.6H2O solution (XT=0.60).
Acknowledgments
To the International Foundation for Science (IFS) for financial support through the E 3066-1 project. To the ANPCyT for financial support through the 06-5066 project. To Dr. C. Añón for the use of CIDCA facilities.
REFERENCES
Cardona, S., C. Schebor, M.P. Buera, M. Karel and J. Chirife, "Thermal stability of invertase in reduced-moisture amorphous matrices in relation to glassy state and trehalose crystallization", J. Food Sci. 62, 105-112 (1997).
Carpenter, J.F., L. Crowe and J. Crowe, "Stabilization of phosphofructokinase with sugars during freeze drying: characterization of enhanced protection in the presence of divalent cations", Biochim. Biophys. Acta. 923, 109-115 (1987).
Cho, S.I., R.L. Stroshine, I.C. Baianu and G.W. Krutz, "Nondestructive Sugar Content Measurements of Intact Fruit Using Spin-Spin Relaxation Time (T2) Measurements by Pulsed 1H Magnetic Resonance", Transactions of the ASAE, 36, 1217 (1993).
Conrad, P.B., J.J. de Pablo, "Computer Simulation of the Cryoprotectant Disaccharide a ,a - Trehalose in Aqueous Solution", J. Phys. Chem. A, 103 4049 (1999).
Cornillon, P, "Characterization of Osmotic Dehydrated Apple by NMR and DSC", Lebensm.-Wiss u Technol, 33 261-266 (2000).
Crowe, L.M., D.S. Reid and J.H. Crowe, "Is trehalose special for preserving dry biomaterials?", Biophysical J. 71, 2087-2093 (1996).
Hartel, R.W., Solid- liquid equilibrium: Crystallization in foods. In: Physical chemistry of foods by Schwartzberg HG, and Hartel R.W., editors. New York: Marcel Dekker. Ch. 2 pp. 47-82 (1992).
Hartel, R.W. and A.V. Shastry, "Sugar Crystallization in Food Products", Critical Reviews in Food Sci. and Nut. 1, 49 (1991).
Jouppila, K. and Y.H. Roos, "Glass Transition and Crystallization in Milk Powders", J. Dairy Sci.., 77, 2907 (1994).
Kedward, C.J., W. Macnaughtan, J.M.V. Blanshard and J.R. Mitchell, "Crystallization kinetics of lactose and sucrose based on isothermal differential scanning calorimetry", J. Food Sci. 63, 192-197 (1998).
Keener, K.M., R.L. Stroshine and J.A. Nyenhuis, "Proton Magnetic Resonance Measurements of Self Diffusion Coefficient of Water in Sucrose Solutions, Citric Acid Solutions, Fruit Juices, and Apple Juice", Transactions of the ASAE, 40, 1633 (1997).
Longinotti, M.P., M.F. Mazzobre, M.P. Buera and H.R. Corti, "Effect of salts on the properties of aqueous sugar systems, in relation to biomaterial stabilization. 2. Sugar crystallization rate and electrical conductivity behaviour", Physical Chemistry and Chemical Physics 4, 533-540 (2001).
Martin, D.R., S. Ablett, A. Darke, R.L. Sutton and M. Sahagian, "Diffusion of Aqueous Sugar Solutions as Affected by Locust Bean Gum Studied by NMR", J. Food Sci. 64, 46-50 (1999).
Mazzobre, M.F. and M.P. Buera, "Combined effects of trehalose and cations on the thermal resistance of b -galactosidase in freeze- dried systems", Biochimica et Biophysica Acta. 1473 337-344 (1999).
Miller, D.P., J.J. de Pablo and H. Corti, "Thermophysical properties of trehalose and its concentrated aqueous solutions", Pharmaceutical Research 14 578-590 (1997).
Official and tentative methods of the American Oil Chemists´Society, Edited by W.E. Link, AOCS, Champaign, Method Cd 10-57 (1974).
Rubin, C.A., J.M. Wasylyk and J.G. Baust, "Investigation of Vitrification by Nuclear Magnetic Resonance and Differential Scanning Calorimetry in Honey: a Model Carbohydrate System", J. Agric. Food Chem. 38 1824- 1829 (1990).
Suzuki, T., K. Imamura, K. Yamamoto, T. Satoh and M. Okazaki, "Thermal stabilization of freeze- dried enzymes by sugars", J Chem Eng of Japan 30, 609-613 (1997). [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ]
Received: September 16, 2001.
Accepted for publication: November 2, 2002.
Recommended by Guest Editors J. Cerdá, S. Díaz and A. Bandoni.














