Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Latin American applied research
Print version ISSN 0327-0793
Lat. Am. appl. res. vol.33 no.2 Bahía Blanca Apr./June 2003
Polyethylenes modified by irradiation and organic peroxide treatment: rheological study
C. J. Pérez, E. M. Vallés, L. M. Quinzani and M. D. Failla
Planta Piloto de Ingeniería Química - PLAPIQUI (UNS-CONICET)
C.C. 717 - 8000 Bahía Blanca, ARGENTINA
e-mail: mfailla@plapiqui.edu.ar
Abstract- A series of polyethylenes was obtained by modification of two commercial high-density polyethylenes. The organic peroxide concentrations and irradiation dosis used are below the critical ones that produce molecular networks. The molecular weights of the polymers increase, and the molecular weight distribution gets wider, as the concentration/dose increases. These results are a consequence of the large molecular weight molecules generated during the chain-linking modification processes. By the time traces of gel begin to appear in the samples, the peroxide modified materials display larger molecular weights and smaller vinyl concentrations than the irradiated ones. The rheological behavior of these materials is analyzed as a function of the molecular structure of the polymers and the concentration/dose used in the modification processes. All the modified polymers show a complex thermo-rheological behavior associated to the presence of long-branched macromolecules. The peroxide treatment generates the polymers with the largest rate of change of flow activation energy.
Keywords- Polyethylene. Rheology. Modification. Irradiation. Peroxide.
I. INTRODUCTION
Over time, the polyethylenes (PEs) have become large-volume commodity resins. The search and analysis of methods to produce new grades of PE with specific properties is an area of increasing interest from an academic and industrial point of view. One way to accomplish this result is by modification of existing commercially produced standard resins. The high-energy ionization radiation and the chemical attack with a peroxide are two of the most used methods to change the molecular structure of PE (Lyons and Weir, 1973; Xantos, 1992; Lambla, 1994; Clough and Shalaby, 1996; Krentsel et al., 1997; Failla et al., 1999; Barkhudaryan, 2000). Both kind of methods are based on the production of macro-radicals that can participate in different chemical reactions. These reactions may involve chain scission and chain linking. In the case of PE prevail the reactions that produce crosslinking, long-chain branching and chain extension (Randall et al., 1983; Bremner et al., 1992; Gloor et al., 1994; Smedberg et al., 1997; Barkhudaryan, 2000). The two mentioned modification processes have been traditionally used mainly to generate PE networks by means of large peroxide concentrations or irradiation dosis (Lyons and Weir, 1973; Bremner et al., 1992; Gloor et al., 1994; Clough and Shalaby, 1996; Smedberg et al., 1997, Kang and Ha, 2000; Palmlof and Hjertberg, 2000). The use of smaller concentrations/dosis than the critical ones to generate networks produces large molecular weight branched PEs that are still thermoplastic.
Small changes in the molecular structure of the polymer produce intense changes in the melt rheological properties as well as in the solid state physical properties. For example, the existence of one long chain branch every ten molecules is sufficient to increment the polymer melt viscosity and tensile strength, the resistance against environment stress cracking, and the improvement of creep properties (Nielsen and Landel, 1994; Lachtermacher and Rudin, 1996; Kim and Yang, 1999; Pérez et al., 2002). Therefore, the identification of the factors that control the modification processes and the understanding of the changes generated in the molecular structure of the polymers are very important issues to study.
In the present work, we compare the rheological response of two high-density polyethylenes (HDPE) to irradiation and peroxide modification. No previous work has performed this type of analysis. The linear polymers have approximately the same concentration of vinyl groups but different molecular weights. The molecular structure of the original and modified polymers was characterized by gel-permeation chromatography (GPC) and infrared spectroscopy (FT-IR). The dynamic rheological properties of the melted polymers were measured in an ample range of frequencies and temperatures. The dependence of the thermal behavior and the flow activation energies with the polymer structure is analyzed.
II. EXPERIMENTAL
The HDPEs used in this study were opportunely supplied by Du Pont de Nemours. The average molecular weights (Mw and Mn) of the original and modified polymers were estimated from GPC (Waters 150-C ALP/GPC) using 1,2,4-trichlorobenzene at 140°C. The system was equipped with a set of 10 m m PL-Gel columns from Polymer Labs having nominal porous size of 106, 103 and 500 Å. The molecular weights of the polymers were estimated following the standard calibration procedure using monodisperse polystyrene samples. The values of Mw and the polydispersity (PD= Mw/Mn) of the initial materials are included in Table 1. The average molecular weights calculated for the modified polymers are underestimated since it is assumed that the polymer molecules are linear. Thus, the GPC characterization allows to perform just a qualitative comparison of the molecular weights as a function of peroxide concentration and irradiation dose. The concentration of vinyl groups of all the materials was calculated by FT-IR (Nicolet 520) from the absorbance at 908 cm-1 using a molar extinction coefficient of 153 l/mol cm (Pérez et al., 2002). The initial concentration of vinyl groups in PEa and PEb is 3,3 10-2 and 3,5 10-2 mol/lt respectively, which corresponds to approximately one vinyl group per molecule in both polymers. According to the polymerization process, the vinyl groups are expected to be in ends of the PE molecules.
Table 1 - Weight-average molecular weights and polydispersity estimated by GPC of all the studied materials

The chemical modification of PE samples was done by previously impregnating the PE powders with different amounts of a peroxide-hexane solution to give the desired final peroxide concentrations after the removal of the solvent. The peroxide used is the 2,5-dimethyl-2,5-di(tert-butylperoxy)-hexane, gently supplied by Akzo Nobel Química S.A. The reactions were performed by press-melting the reactive blends at 170° C for 20 min between the plates of an hydraulic press. The half-life time of the peroxide at 170°C is of approximately three minutes. Samples of both polymers in the form of 1 mm thick films were irradiated under vacuum by exposing them to g-rays generated by a 60Co source at room temperature. After irradiation the samples were annealed at 150° C for 4 h to eliminate the remaining free radicals (Lyons and Weir, 1973).
The modified polymers are identified by the name of the original polyethylene (PEa or PEb) followed by a number that distinguishes the concentration of peroxide, in ppm, or the doses of irradiation, in kGy, used in the modification processes, and a letter associated with the process (p: peroxide, i: irradiation). For example, PEb-15i is the sample of PEb that has been radiated to a dose of 15 kGy.
The rheological characterization of the melted polymers was carried in a rotational rheometer (RDA-II from Rheometrics) using parallel plates of 25 mm diameter. The elastic modulus, G, and viscous modulus, G, of the polymers were measured in small-amplitude oscillatory shear flow as a function of frequency (from 0.1 to 400 s-1) and temperature (from 140 to 200°C). The tests were performed under nitrogen atmosphere using small amplitudes. Strain sweep experiments were previously carried out to determine the range of amplitudes that allow to work in the linear viscoelastic regime. The dynamic viscosity (h=G/w) and the phase angle (d = tan-1 G/G) are calculated rheological parameters also used in this paper. No measurable degradation was observed in the tested samples.
III. RESULTS AND DISCUSSION
Figure 1 displays the normalized chromatograms of the studied polymers and the corresponding modified materials obtained by peroxide attack and irradiation. The materials are completely soluble below the following levels of dosage and peroxide concentration: 30 kGy and 2000 ppm for PEa, and 20 kGy and 500 ppm for PEb. Gel fractions begin to appear when larger concentrations/dosis are used. Figure 1 shows that a gradual increase in the width of the chromatograms and a shift of the maximum towards lower elution volumes occurs when the peroxide concentration increases. These effects are produced by the new species (of molecular weight larger than the original ones) that appear as the modification degree increases. The modification with peroxide seems to produce a larger change in the molecular structure than the irradiation process. The chromatograms of the irradiated samples also display an increase of the width and a shift of the maximum towards lower elution volumes, but those changes are much less noticeable than in the case of the peroxide modification. The peroxide attack produces a larger decrease in the relative concentration of low molecular weight species as well as a much larger increase in the relative concentration of species at the high molecular weight tail of the distribution. These results indicate that there exist some differences between the two modification processes under the experimental conditions adopted in this study. The weight-average molecular weight and polydispersity of the polymers are listed in Table 1. When analyzing the values, it must be taken into account that the difference between the real molecular weights and the calculated ones increases as the concentration/dose increases because they are estimated assuming that the polymer molecules are linear.
Figure 1.Chromatograms of the original polyethylenes and the modified materials.
In a previous work it has been demonstrated that the vinyl groups play a very important role in the peroxide modification process (Pérez et al., 2002). According to the results presented in Fig. 1, the role played by the vinyl group in the irradiation process is less significant than in the peroxide modification process. Figure 2 confirms this conclusion. In this figure, the concentration of vinyl groups of all the polymers (expressed relatively to the vinyl concentration of the original polymers) is plotted against the peroxide concentration and the irradiation dose. The maximum values of the x-axis, that is, 2000 ppm and 30 kGy, were selected because PEa-2000p and PEa-30i are materials whose rheological characterization show evidence of the presence of gels even though no traces of gel were found. Gel fractions appear by selective extraction with solvent in the case of PEa2500p and PEa40i. It may be observed in Fig. 2 that, by the time gel fractions appear in the irradiated polymers, a relatively large concentration of vinyl groups are still present in the samples. On the other hand, the concentration of vinyl groups has reduced to very low values by the time the polymers modified with peroxide show traces of insoluble material. This result indicates that the vinyl groups have an important participation in the chemical events that occur in the case of peroxide modification before the gel point. This observation agrees with the GPC results and the larger amount of high molecular weight molecules found in the chromatograms of the peroxide modified materials.
In the irradiation process, the probability of a molecule to participate in a chain-linking reaction is given by its size, i.e., its molecular weight. Consequently, this process produces large molecules that grow very rapidly in size as the dose increases, but with no significant shift of the maximum because the low molecular weight tail is little affected. In the case of modification by peroxide treatment, the probability of the molecules to react is also given by its size, but the presence of vinyl groups enhances the possibility of the chain ends to participate in the reaction. For this reason the chromatograms of the peroxide modified PEs show more notable shifts of the maximums towards high molecular weights and reductions of the low molecular weight tails.

Figure 2. Concentration of vinyl groups of the modified polymers relative to the one of the original PEs as a function of peroxide concentration and irradiation dose.
Figures 3 and 4 show the dynamic viscosity, h ¢ (w ), and the elastic moduli, G¢ (w ), data corresponding to all the materials at 160° C. The modified materials show a progressive increase of both the viscous and elastic moduli as the concentration or dose augments. This effect is due to the increasing amount of large macromolecules that are formed by the chain-linking reactions. The effect is more noticeable in the PE samples modified with peroxide in accordance with the results of molecular characterization by GPC.
Comparing Figs. 3 and 4, it can be observed that, in the case of the PE of larger molecular weight, a much smaller concentration of peroxide is necessary to obtain equivalent changes in the rheological parameters. PEb, which has a Mw that is 1.57 times that of PEa, forms very large macromolecules with a very small amount of peroxide. For example, according to Figs. 3 and 4, the addition of 65 ppm of peroxide to PEb is sufficient to produce a relative increase in the rheological parameters similar to the addition of approximately 500 ppm in the case of PEa. That is not the case with the irradiated materials. Similar dosis produce similar relative effects in both polymers. See, for example, the relative position of the dynamic moduli curves of PEa-15i to PEa and those of PEb-15i to PEb. The results emphasize the important role played by the vinyl groups in the peroxide modification process.
The linear viscoelastic parameters of the polymers were also analyzed as a function of temperature in the range from 140 to 200°C. The curves of the dynamic moduli of PEa have similar behavior with frequency (see Fig. 5) and they may be shifted according to the time-temperature superposition principle to build master curves of the dynamic material parameters. PEb, on the other hand, has no simple thermo- rheological behavior, as it may be appreciated in Fig. 5. The curves of the PEb data cannot be superposed to obtain master curves suggesting the presence of some long chain branches in the original material (Pérez et al., 2002). In the case of PEb, two pseudo-master curves may be built from superposing the viscoelastic data at low and high frequencies respectively. All the modified materials show thermo-rheologically complex behavior. As in the case of PEb, the time-temperature superposition principle cannot be used with these PEs in the whole range of frequencies covered, although pseudo-master curves may be built from superposing the viscoelastic data in smaller ranges of frequencies. This procedure allows, for example, to further analyze the temperature dependence of the low-frequency data, which are affected by the large relaxation processes and are sensitive to the large-scale molecular structure. The thermo-rheological complexity of the modified polymers is a consequence of the long chain branches generated in the polymer molecules during the irradiation and peroxide modification process.
Figure 3. Elastic modulus (filled symbols) and dynamic viscosity (empty symbols) of the original PEa and the modified samples at 160°C
Figure 4. Elastic modulus (filled symbols) and dynamic viscosity (empty symbols) of the original PEb and the modified samples at 160°C.
The factors used to shift the data curves to build master- and pseudo-master-curves in the time scale, aT, show an Arrhenius type of dependence with temperature, i.e. ln aT » D H / R T, where DH is the flow-activation energy and R is the universal gas constant. Figure 6 shows the values of DH/R of all the studied polymers. The flow activation energy of PEa, 3120 K, is in accordance with previously reported values for linear PEs (Pérez et al., 2002). In the case of PEb and the modified polymers, the flow activation energies displayed in Fig. 6 are the ones that describe the temperature dependence of the slower relaxation processes, i.e. those that dominate the response of the polymers at small frequencies and that may be associated to the large-scale dynamics of the molecules. Only in the case of PEb, PEa-5i and PEb-5i, it was possible to shift the curves and superpose them in the range of large fre- quencies (w > ~10 s-1). A value of ~4200 K was measured for DH/R in this range of frequencies for these three polymers. These values describe the temperature

Figure 5. Frequency dependence of the phase angle of PEa and PEb as a function of temperature.
dependency of relaxation processes that are several orders of magnitude faster than the large ones previously mentioned. These relaxation processes are associated to molecular segments much smaller than the size of the hole molecule but much larger than the distance between entanglements.
The DH values determined from the low frequency range data reflect the complexity of the molecular structure generated by the chain linking reactions during the modification processes. The flow activation energy increases as the peroxide concentration and irradiation dose increase. But the rate of increase of DH is larger in the family of peroxide modified materials than in the case of the irradiated samples. Larger values of flow activation energy are reached by the materials treated with peroxide compared to the irradiated samples as they approach the gel point. This result is in agreement with the GPC and FT-IR observations previously commented.
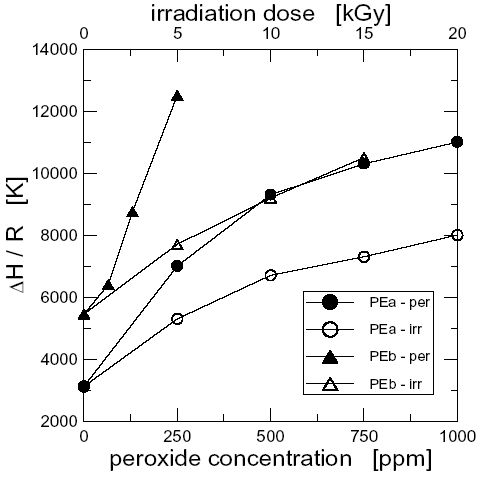
Figure 6. Flow activation energy of the polymers as a function of peroxide concentration and irradiation dose.
It is interesting to notice that the flow activation energy of the polymers modified with the smaller concentrations of peroxide and irradiation dose (PEa-250p, PEa-5i, PEa-10i and PEb-65p) are similar to the flow activation energy of the low density polyethylene, LDPE (DH/R » 6600 K (Wasserman and Graessley, 1996)). This result suggests that, from the point of view of the thermal response, the branched molecular structure obtained in these cases may be similar to the conventional branched structure of PEs. The larger values of DH observed in the materials modified with larger dosis/concentrations suggest a much more complex molecular structure which it may be of the type of branches-on-branches.
IV. CONCLUSIONS
The results of the present study show that the molecular structure and molecular weight distribution of modified PEs depend on the modification process used and on the initial molecular weight of the polymer.
The chromatographic and rheological data suggest that the degree of modification of PE with peroxide depends on the molecular weight and the concentration of vinyl groups of the original polymer. All the molecules of the studied materials, which have approximately one vinyl group per molecule, have similar probability to react. That is the reason why the chromatograms of the peroxide modified PEs show a shift of the maximum towards high molecular weights and a notable reduction of the low molecular weight tails. The irradiation process also produces large molecules that keep increasing their size as the dose increases, but no significant shift of the maximum is observed in the chromatograms. The results agree with the generally accepted theory that the possibility to generate a chain-link by irradiation is proportional to the molecular weight of the material and it is slightly affected by the presence of vinyl groups.
The PEs generated by peroxide modification have larger viscous and elastic moduli than the ones of equivalent molecular weight obtained by irradiation. This is because, in the irradiated samples, very large molecules coexist with small ones that produce a solvent-like effect in the flow properties of these polymers.
All the modified materials are thermo-rheologically complex polymers, as a consequence of the long-chain branched macromolecules generated during the modification processes. These complex structures generate relaxation processes in the polymers with much longer relaxation times and much larger flow activation energies than those of the molecules with linear backbones.
Acknowledgments
The authors wish to thank CONICET, ANPCyT and Universidad Nacional del Sur for the financial support.
REFERENCES
Barkhudaryan, V.G., "Alterations of molecular characteristics of polyethylene under the influence of g radiation", Polymer 41, 2511-2520 (2000).
Bremner, T., A. Rudin and S. Haridoss, "Effect of polyethylene molecular structure on peroxide crosslinking of LDPE", Pol. Eng. Sci. 32, 939-943 (1992).
Clough, R.L. and S.W. Shalaby, "Irradiation of polymers: Fundamentals and technological applications", ACS Symp. Series, Vol. 620 (1996).
Failla, M.D., E.M. Vallés and B.J. Lyons, "Effect of initial crystallinity on the response of high-density polyethylene to high-energy radiation", J. Appl. Polym. Sci., 71, 1375-1384 (1999).
Gloor, P.E., Y. Tang, A. Kostanska and A.E. Hamielec, "Chemical modification of polyolefins by free radical mechanism: A modeling and experimental study of simultaneous random scission, branching and crosslinking", Polymer, 35, 1012-1030 (1994).
Kang, T.K. and C.S. Ha, "Effect of processing variables on the crosslinking of HDPE by peroxide", Polymer Testing, 19, 773-783 (2000).
Kim, Y.C. and K.S. Yang, "Effect of peroxide modification on melt fracture of LLDPE during extrusion", Polymer J., 31, 579-584 (1999).
Krentsel, B.A., Y.V. Kissin, V.I. Kleiner and L.L. Stotskaya, Polymers and Copolymers of Higher a-Olefins. Chemistry, Technology, Applications, Hanser/Gardner Publ., Munich (1997).
Lachtermacher, M.G. and E. Rudin, "Reactive processing of LLDPEs with peroxide in counter-rotating nonintermeshing twin-screw extruder, I to IV", J. Appl. Polym. Sci., 58, 2077-2094 and 2433-2440 (1995); 59, 1213-1221 and 1755-1785 (1996).
Lambla, M.. "Reactive extrusion: A new tool for the diversification of polymeric materials", Macromol. Symp., 83, 37-58 (1994).
Lyons, B.J., F.E. Weir, In: The radiation Chemistry of Macromolecules, Vol. II, M. Dole (edit.), Academic Press, New York, Ch. 14 (1973).
Nielsen, L. and R. Landel, Mechanical Properties of Polymers and Composites, M. Dekker, NY (1994).
Palmlof, M. and T. Hjertberg, "Chemical and mechanical changes in poly(ethylene-co-1,9-decadiene) following crosslinking induced by peroxide", Polymer, 41, 6497-6505 (2000).
Pérez, C.J., G.A. Cassano, E.M. Vallés, M.D. Failla and L.M. Quinzani, "Rheological study of linear high density polyethylenes modified with organic peroxide", Polymer, 43, 2711-2720 (2002).
Randall, J.C., F.J. Zoepfl and J. Silverman, "A 13C NMR study of radiation. Induced long chain branching in polyethylene", Macromol. Chem., Rapid Commun., 4, 149-157 (1983).
Smedberg, A., T. Hjertberg and B. Gustafsson, "Crosslinking reactions in an unsaturated low density polyethylene", Polymer, 38, 4127-4138 (1997).
Xantos, M, Reactive Extrusion, Hanser Pub, NY (1992). [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ]
Received: September 16, 2001.
Accepted for publication: August 15, 2002.
Recommended by Guest Editors: J. Cerdá, S. Díaz and A. Bandoni.














