Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Latin American applied research
versión impresa ISSN 0327-0793
Lat. Am. appl. res. v.33 n.2 Bahía Blanca abr./jun. 2003
Simulation of an industrial packed column for reactive absorption of CO2
C. Gómez, D. O. Borio and N. S. Schbib
Planta Piloto de Ingenieria Quimica - PLAPIQUI (UNS-CONICET)
Camino La Carrindanga, Km 7. - Casilla de Correo 717. - 8000 Bahía Blanca. - ARGENTINA.
e-mail: sschbib@plapiqui.edu.ar
Abstract - The steady-state simulation of a reactive absorption column is presented. The absorber is used in a large-scale ammonia plant to remove CO2 from the process gas stream. To enhance the absorption process, high pressures and low temperatures are commonly used (T = 45-80 °C, P = 30-40 bar). At the outlet of the absorber, the CO2 content in the process gas must be reduced to less than 500 ppm (dry basis) to avoid an excessive temperature rise in the methanation reactor (downstream of the absorption section). To represent the gas-liquid system, a rigorous mathematical model based on the two-film theory is considered. The heat effects are taken into account. The behaviour of different process variables for a reference operating condition is analyzed. The influence of changes in some operating variables is evaluated.
Keywords - Reactive Absorption Of CO2. Packed Columns. Ammonia Synthesis. MDEA.
I. INTRODUCTION
Reactions of carbon dioxide with amines have been extensively studied during the last 20 years because of their industrial importance for the natural gas, petroleum chemical plants, and ammonia industries for removal of CO2 from gas streams (Pintola et al., 1993). In an ammonia synthesis plant, the CO2 must be removed from the process gas to avoid the poisoning of the ammonia synthesis catalyst. The CO2 removal represents 10% of the capital and operating costs (Meissner III and Wagner, 1983).
The absorption in alkanolamine solutions is the commercially most important process for the removal of CO2 from synthesis gas for ammonia production. A wide variety of chemical solvents are used, e.g. MEA (monoethanolamine), DEA (diethanolamine), MDEA (methyl diethanolamine), activated MDEA (mixture MDEA - piperazine), and recently AMP (2-amino-2-methyl-1-propanol) (Ko and Li, 2000).
The rigorous simulation of a packed column used industrially for reactive absorption of CO2 using a MDEA solution is presented in this paper, aiming to obtain a useful tool for optimisation purposes.
Figure 1 shows a scheme of the industrial column. The CO2 is removed from the process gas stream by counter-current absorption in two stages. In the lower part of the absorber, solution regenerated by flashing (semi-lean solution) is used for bulk CO2 removal. In the upper part of the absorber, solution regenerated by stripping (lean solution) is used for scrubbing (Meissner III and Wagner, 1983).
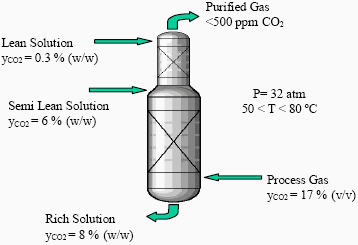
Figure 1. Scheme of the industrial absorption column
II. MATHEMATICAL MODEL
The two-film theory is adopted to represent the gas-liquid system (Froment and Bischoff, 1990, De Leye and Froment, 1986). The main chemical reactions are:
![]() (1)
(1)
![]() (2)
(2)
The most common situation is that the absorption of CO2 in MDEA is accompanied by very fast reactions (Hatta Number > 3). Consequently, the reactions are assumed to be completed in the liquid film (![]() = 0). The pseudo-first order kinetic expressions proposed by Ko and Li (2000) are adopted.
= 0). The pseudo-first order kinetic expressions proposed by Ko and Li (2000) are adopted.
Steady-state conditions and plug flow is assumed for the gas phase, leading to a set of Ordinary Differential Equations (ODEs).
Mass balance for CO2 in the gas phase
 | (3) |
Total molar flow in the gas phase
| (4) |
the interfacial flux of CO2 per unit gas-liquid area (![]() ) for a pseudo first-order reaction is given (Doraiswamy and Sharma, 1984) by
) for a pseudo first-order reaction is given (Doraiswamy and Sharma, 1984) by
 | (5) |
where the overall reaction rate constant (k) has the following expression
![]() (6)
(6)
The gas-liquid equilibrium of CO2 at the interface is expressed by:
![]() (7)
(7)
While the interfacial flux in the gas film is given by
| (8) |
Data reported by Hani et al. (1989) are used to evaluate the solubility and diffusivity of CO2 in amine solutions. The gas and liquid-side mass transfer coefficients and the interfacial area of the packing are estimated from the correlations proposed by Onda et al., (1968a, b), Linek et al., (1995) and Laurent and Charpentier (1974).
The liquid flowrate, the concentrations of amine and ionic products and the temperature are updated by means of algebraic equations at each increment (D z) used in the integration of the ODE´s:
Total mass balance in the liquid phase
 | (9) |
Mass balance for component j in the liquid phase
 | (10) |
Energy balance
 | (11) |
To evaluate the absorption and reaction heats the experimental data of Jou et al. (1982) were taken into account. In these data the heats effects were considered together.
The numerical solution of the problem is obtained following an iterative process. The algebraic equations are solved by means of a Quasi-Newton Method and the integration of the ODEs along the axial coordinate is carried out using a Gear algorithm.
III. RESULTS AND DISCUSSION
A. Reference Case
Figure 2 shows the variation of the partial pressure of CO2 along the column, for the operating conditions selected as the reference case. As it can be seen, pCO2 drops from a value of around 5.6 bar (at the bottom) to an outlet value of 0.013 bar, i.e, the outlet CO2 content (400 ppm. v/v) is lower than the maximum allowable value. For these operating conditions, more than 99.7 % of the CO2 fed to the column is absorbed by the amine solution.

Figure 2. Reference case: partial pressure of CO2 and MDEA concentration along the axial coordinate
The reaction of the absorbed CO2 with the amine leads to a decrease in the concentration of unreacted MDEA from the top (inlet) to the bottom. The discontinuity observed for the curve of MDEA corresponds to the column height where the semi-lean solution is admitted. At these axial position (z* = 0.56), the entering semi-lean solution has a lower MDEA concentration than the liquid leaving the upper section of the column.
The axial profiles for the total gas flowrate (F) and total liquid flowrate (L) are shown in Fig, 3. Since the process gas to be purified has an important content of CO2, the gas flowrate decreases around 17 % (v/v) from the bottom (inlet) to the top of the column. It is interesting to note that from the total amount of CO2 being removed, the most important fraction is absorbed in the lower section of the column. The rest of the CO2 is absorbed by the lean solution at the top of the absorber to obtain the desired purity. The lean and semi-lean solutions have 0.3% and 6% w/w of CO2 respectively, which is present as reaction product. ![]() .
.

Figure 3. Reference case: gas and liquid flowrates along the axial coordinate
The L axial profile has an opposite trend to that observed for F, because of the countercurrent configuration. The sudden increase in the liquid flowrate at z*=0.56 is caused by the admission of the semi-lean solution, whose flowrate is around 5 times higher than that of the lean solution.
The temperature variation along the absorber is shown in Figure 4. At the upper section of the column, the temperature increases around 5°C due to the exothermic nature of the reactive absorption process. The discontinuity in the thermal profile is caused by the semi-lean solution stream, which has a higher thermal level than the liquid leaving the upper section. After a further increase of approximately 12°C in the lower section, the MDEA (rich) solution leaves the bottom of the absorber to be regenerated.
The Hatta number along the column changes between 3.5 (top) and 5.2 (bottom). This range confirms that the absorption of CO2 in MDEA is accompanied by very fast reactions such as was assumed in the mathematical model (item 2)

Figure 4. Reference case: temperature along the axial coordinate
B. Influence of the process gas flowrate
Figures 5 and 6 show the parametric sensitivity of the model with respect to changes in the process gas flowrate. This situation is representative of changes in the ammonia plant capacity. Figure 5 shows the CO2 molar fraction in the process gas along the axial coordinate, for different values of the total gas flowrate.

Figure 5. Molar fraction of CO2 in the process gas along the axial coordinate, for three different values of the process gas flowrate
As expected, as the F value is increased the content of CO2 increases at all the axial positions. Consequently, the CO2 content at the top of the column (CO2 slip) can exceed the maximum allowable level of 500 ppm. This undesired situation is illustrated in Fig. 5 for the case of a F value 10 % higher than the reference flowrate (see the enlarged area). The increase in the CO2 slip should be controlled (e.g., by increasing the liquid flowrates or adapting the temperature of the lean solution) to limit the temperature rise in the methanation reactor, downstream of the absorption section.
The influence of the process gas flowrate on the absorber temperature is shown in Figure 6. The temperature at the top of the column (z*=0) is constant because it is defined by the inlet temperature of the lean solution. The overall temperature rise along the column increases with the value of F. This is a consequence of the higher heat generation rates which is associated to the higher absorption rates of CO2. The temperature is particularly affected in the scrubbing section of the column (0< z*< 0.56), because the liquid flowrate is notably lower than that of the bottom section (z* > 0.56).

Figure 6. Temperature along the axial coordinate for three different values of the process gas flowrate
C. Influence of the pressure:
The influence of the total pressure on the CO2 content in the process gas is shown in Figure 7. As it can be seen, a decrease in the pressure of the column (which may be caused upstream the absorber) causes a lower partial pressure of CO2 in the gas phase, then the equilibrium concentration of CO2 (![]() ) decreases at all the axial positions. As a consequence, lower reaction rates take place in the liquid film and the CO2 slip in the process gas stream increases. In fact, for the case of P =25 bar the column does not satisfy the specification of 500 ppm. in the purified process gas. The change in the total pressure also affects the absorber temperature and higher temperature rises occur as P increases.
) decreases at all the axial positions. As a consequence, lower reaction rates take place in the liquid film and the CO2 slip in the process gas stream increases. In fact, for the case of P =25 bar the column does not satisfy the specification of 500 ppm. in the purified process gas. The change in the total pressure also affects the absorber temperature and higher temperature rises occur as P increases.

Figure 7. Molar fraction of CO2 in the process gas along the axial coordinate, for three different values of the pressure
V. CONCLUSIONS
The detailed modeling and simulation of an industrial column for CO2 absorption has been carried out. The mathematical model is a useful tool to evaluate the steady-state behavior of the absorber under different operating conditions. This rigorous model can be incorporated as an external module in a commercial (or ad-hoc) simulator of the whole CO2 removal section, to obtain a more realistic representation of the complex phenomenon of reactive absorption.
Acknowledgements
The authors are grateful to Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) and Universidad Nacional del Sur (FOMEC Program) of Argentina.
NOMENCLATURE

Greek symbols

Subscripts

Superscripts
![]()
REFERENCES
De Leye, L. and Froment, G., "Rigorous simulation and design of columns for gas absorption and Chemical Reaction - I Packed columns", Comp. & Chem. Eng., 10 (5), 493-504 (1986). [ Links ]
Doraiswamy, L.K. and Sharma, M.M., "Heterogeneous reactions: Analysis, examples, reactor design", 2. In Fluid-fluid-solid reactions. New York, Wiley (1984). [ Links ]
Froment G.F. and K.B. Bischoff, Chemical Reactor Analysis and Design. Wiley, New York (1990). [ Links ]
Hani Al-Ghawas, D. Hagewiesche, G. Ruiz-Ibañez and O. Sandall, J. Chem. Eng. Data, 34, 385-391 (1989). [ Links ]
Ko Jiun-Jie and Li Meng-Hui, "Kinetics of absorption of carbon dioxide into solutions of N-methyldiethanolamine + water", Chem. Eng. Sci., 55, 4139-4147 (2000). [ Links ]
Jou F., Mather A., and Otto F., "Solubility of H2S and CO2 in Aqueous Methyldiethanolamine Solutions", Ind. Eng. Chem. Process Des. Dev., 21, 539-544 (1982). [ Links ]
Laurent A. and Charpentier J., "Aires interfaciales et Coefficients de Transfert de Matiere dans les Divers Types d´Absorbeurs et de Reacteurs Gaz-Liquide", The Chem. Eng. Journal, 8, 85-101 (1974). [ Links ]
Linek V., Sinkule J., Brekke K., "A Critical Evaluation of the Use of Absorption Mass Transfer Data for the Design of Packed Distillation Columns", Trans IChemE, 73, part A, 398-405 (1995). [ Links ]
Meissner R.E. III and Ulrich Wagner, "Low -energy Process Recovers CO2", Oil and Gas Journal, 55-58 (1983). [ Links ]
Onda K., Takeuchi H. and Okumoto Y., "Mass Transfer Coefficients between Gas and Liquid Phases in Packed Columns", J. Chem. Eng. Japan, 1, 1, 56-62 (1968a). [ Links ]
Onda K., Sada E., Takeuchi H., "Gas Absorption with Chemical Reaction in Packed Columns", J. Chem. Eng. Japan, 1, 1, 62-66 (1968b). [ Links ]
Pintola T., Tontiwachwuthikul P. and Meisen A. "Simulation of Pilot Plant and Industrial CO2 – MEA absorbers", Gas Separation & Purification, 7, 1, 47-52 (1993). [ Links ]
Received: September 16, 2001.
Accepted for publication: November 02, 2002.
Recommended by Guest Editors: J. Cerdá, S. Díaz and A. Bandoni.














