Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Latin American applied research
versión impresa ISSN 0327-0793
Lat. Am. appl. res. v.33 n.3 Bahía Blanca jul./sept. 2003
Multicomponent ion exchange isotherms in NaX zeolite
M. A. S. D. Barros1, I. F. Araújo Jr.1, P. A. Arroyo1, E. F. Sousa-Aguiar2,3, C. R. G. Tavares1
1 Chemical Engineering Department, State University of Maringá, Brazil, angelica@deq.uem.br
2 DPO, Federal University of Rio de Janeiro, 3 CENPES/PETROBRAS, Brazil.
Abstract ¾ In this work the ion exchange isotherms of Cr3+, Mg2+, Ca2+ and K+ in single solutions and in binary mixtures (Cr/K, Cr/Ca, Cr/Mg) using NaX zeolite at 30oC, 45oC and 60oC were reported. NaX isotherms were very favorable for all metal cations studied. The respective isotherms had some differences in shape, a consequence of competition for the exchange sites. The Kielland plots of all systems were non linear, which is a characteristic of sites of different energies involved in the exchange process. Temperature influenced the ion exchange as it could alter the hydration sphere of the in-going ions. Consequently, differences in thermodynamic properties and also in the selectivity were seen. It was concluded that NaX did not have a pronounced selectivity towards chromium.
Keywords ¾ Ion Exchange. Isotherm. Zeolite.
I. INTRODUCTION
Heavy metals are not biodegradable and tend to accumulate in living organisms, causing various diseases and disorders. An alternative system of cation removal from these wastewaters is represented by ion exchange in zeolites. In this work it was used the synthetic zeolite X. It has three types of cavities: the supercages, the sodalite and the hexagonal prisms. As a consequence, different sites called SII, SI and SI (Giannetto et al., 2000) are observed. The site SII is located in the supercages whereas SI is located in the sodalite cages and SI in the hexagonal prisms. SII is the easiest site to be reached and SI the most difficult one. Ion exchange equilibrium has been studied for a lot of divalent and trivalent cations (Keane, 1996; Maes and Cremers, 1975). However, concerning chromium exchange, few studies have been reported in synthetic zeolites (Tagami et al., 2001) and relatively little is known about the combined effects of two or more metal ions and their simultaneous removal. In our ion exchange studies, equilibrium isotherms of single and multicomponent exchange with Cr3+ and the main cations present in industrial wastewater (Ca2+, Mg2+ also K+) have been studied. The resultant findings of binary exchange (Cr-NaX, Ca-NaX, Mg-NaX and K-NaX) and ternary exchange (Cr/Ca-NaX, Cr/Mg-NaX, Cr-K-NaX) are presented in this paper.
II. EXPERIMENTAL
A. Materials
The starting zeolite was a very high crystalline NaX, its unit cell composition in dry basis was Na81(AlO2)81(SiO2)111 corresponding to a cation exchange capacity of 5.96 meq/g. In order to obtain, as far as possible, the homoionic sodium form, the zeolite, as received, was contacted four times with 1 mol/L solutions of NaCl at 60oC with a zeolite/solution ratio of 1:10. The zeolite was then washed each time with 2 L of hot deionised water and oven-dried at 100oC. The reagent-grades CrCl3.9H2O, MgCl2.6H2O, CaCl2.2H2O and KCl were mixed with deionised water to prepare 15 meq/L solutions for single ion exchange. For binary solutions both reagents were used (15 meq/L each one) in order to produce initial solution Cr/Mg, Cr/Ca and Cr/K, with a constant equivalent ratio 1:1. The ion exchange reactions were carried out by weighting suitable quantities of NaX zeolite (0.01g up to 1.50g) in 35-mL flasks containing 20 g of the salt solution and letting the system equilibrate in water bath shaker at 30, 45 and 60oC for a period of four days for binary isotherms and seven days for ternary isotherms. Such equilibrium time had been previously checked and it was concluded that it was sufficient enough to attain equilibrium between the zeolite and the solution phase. After the corresponding equilibrium time, the flasks were removed from the constant-temperature bath and the solid as well as solution phases were rapidly separated by filtration. The ion-exchange studies were executed without pH control in order to avoid the addition of another exchangeable cation. In all sample flasks the pH was between 3.0 and 4.0, which indicated that dealumination of the zeolite or precipitation of hydroxides may be neglected and the isotherms represented only the sorption mechanism in the zeolite.
The cation contents in the solution phase were determined by atomic absorption spectrometry using a Varian SpectrAA10-Plus spectrometer.
B. METHODS
The isotherm corresponding to binary exchange is normally expressed as XAS plotted against XAZ at a constant total ion concentration of the solution where XAS and XAZ are the equivalent fractions of ion A in solution and in the zeolite, respectively. When there is a ternary exchange system, where the in-going cations A and C and the out-going cation B are involved, the isotherms present XAS plotted against XAZ and XCS against XCZ. Generically the equivalent fractions are denominated as XiS for ions in solution and XiZ for ions in the zeolite.
The isotherm for ion exchange is calculated by:
 | and | 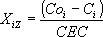 | (1) |
where Ci stands for the final concentration of the cation A or C in the solution. CO stands for the initial concentration of the in-going cation present in a single solution and the sum of the initial concentration of both in-going cations when in bicomponent solution. Coi is the initial concentration of cation i. CEC is the cation exchange capacity of the respective zeolite mass. Isotherms provide information on the zeolite selectivity but they do not give information about the type of exchange sites although it is necessary for a better understanding of the mechanism of the ion-exchange phenomenon. However, with these data, some parameters may be calculated, for example the selectivity coefficient as described in Tagami et al. (2001). In order to obtain  the mean activity coefficients of individual strong electrolyte for pure and multicomponent solutions (gi(S)) must be known. These parameter can be obtained using Meissners method described in Zemaitis et al. (1986) and Meissner and Kussik (1972). The Kielland plot, obtained by
the mean activity coefficients of individual strong electrolyte for pure and multicomponent solutions (gi(S)) must be known. These parameter can be obtained using Meissners method described in Zemaitis et al. (1986) and Meissner and Kussik (1972). The Kielland plot, obtained by  , may provide us with information regarding the exchange mechanism. The Kielland plots are merely plots of log
, may provide us with information regarding the exchange mechanism. The Kielland plots are merely plots of log  versus XiZ, where i is any in-going ion, A or C. Non-linear plots are frequently found in X zeolites due to distinct groups of exchange sites participating in the ion exchange reaction (Maes and Cremers, 1975). As in X zeolites sites SII, SI and SI are observed and it is easy to conclude that, because of their location in the framework, these sites have differences in the exchange energy. The thermodynamic equilibrium constant for ion exchange (Ki) is estimated from the Kielland plots. The standard enthalpy of exchange (DHo) and the standard free energy of exchange (DGo) are estimated from the temperature dependence of Ki. In cases of incomplete ternary exchange, a normalization procedure is required in which the experimental XAZ values are multiplied by a normalization factor f = 1/XAZmax, where XAZmax represents the maximum exchange level observed (Barrer and Klinowski, 1972). In order to calculate
versus XiZ, where i is any in-going ion, A or C. Non-linear plots are frequently found in X zeolites due to distinct groups of exchange sites participating in the ion exchange reaction (Maes and Cremers, 1975). As in X zeolites sites SII, SI and SI are observed and it is easy to conclude that, because of their location in the framework, these sites have differences in the exchange energy. The thermodynamic equilibrium constant for ion exchange (Ki) is estimated from the Kielland plots. The standard enthalpy of exchange (DHo) and the standard free energy of exchange (DGo) are estimated from the temperature dependence of Ki. In cases of incomplete ternary exchange, a normalization procedure is required in which the experimental XAZ values are multiplied by a normalization factor f = 1/XAZmax, where XAZmax represents the maximum exchange level observed (Barrer and Klinowski, 1972). In order to calculate  , all XAZ experimental values were normalized. For multicomponent ion exchange the selectivity coefficient and the thermodynamic properties could be obtained as for binary ion exchange. In this case, normalized XiZ values could be obtained choosing the maximum XiZ for the in-going cations. D Go and D Ho values have been calculated for the isotherms from the numerical integration of Kielland plots of each in-going ion.
, all XAZ experimental values were normalized. For multicomponent ion exchange the selectivity coefficient and the thermodynamic properties could be obtained as for binary ion exchange. In this case, normalized XiZ values could be obtained choosing the maximum XiZ for the in-going cations. D Go and D Ho values have been calculated for the isotherms from the numerical integration of Kielland plots of each in-going ion.
III. RESULTS AND DISCUSSION
All isotherms of Cr3+, Mg2+, Ca2+ and K+ at 30, 45 and 60oC for binary ion exchange are shown in Fig. 1 a, b, c and d. It may be observed that the isotherms clearly displayed the very high selectivities for all cations. Different convexly upward curvatures were obtained as a consequence of different degrees of exchange promoted by the size and charge of the cations, temperature of the system and cation-framework interaction (Giannetto et al., 2000). Concerning the isotherms shown in Fig. 1-a, it was observed that the exchange limit for calcium at 30oC reached slightly higher XCaZ values than for the other temperatures. At 30oC the interactions between Ca2+ and the zeolite were supposed to be really important. The pronounced convexly curvature for the Ca-NaX isotherm indicated a very strong preference for calcium over sodium especially at low calcium loadings. After the inflexion point (XAZ » 0.8) the curvature was concavely upward reflecting some difficulty of the in-going ion in diffusing and being exchanged in the hexagonal prisms. At 45oC it could be seen that calcium ions reached less hexagonal prisms, as XCaZ values were lower than at 30oC. Finally at 60oC, the exchange terminated at 82% of the theoretical exchange capacity, suggesting that no Ca2+ was located in the hexagonal prisms. The non-favorable temperature influence for Ca-NaX systems was already observed at 25oC.
a)

b)

c)

d)

Fig. 1: Single exchange isotherms in NaX for a) Ca2+, b) K+, c) Mg2+ and d) Cr3+ at (¨) 30oC, (£) 45oC, (D) 60oC
At this temperature a complete Ca exchange was achieved and at 50oC approximately 80% of the Na+ ions were replaced by these divalent ions (Sherry, 1968).It can be seen in Fig. 1-b that an increase in temperature promoted less exchanges in K-NaX systems. The isotherms were almost straight lines, possibly due to a small affinity of the zeolite with an in-going cation of the same charge as the out-going ion Na+. It is interesting to note in Fig. 1-c that the influence of temperature was just the opposite of when magnesium exchange was considered. The progress of Mg2+ exchange was clearly enhanced as the temperature was increased. Probably at 30oC Mg2+ ions were exchanged only in the sites located in the large cages and at higher temperatures, all sites were progressively occupied by the entering cation. It was worth to observe some overexchanged values (XMgZ > 1) at 45 and 60oC, which were due to some multilayer adsorption. This phenomenon became increasingly significant as the monolayer, which corresponds to the ion-exchange process, was more and more crowded, that is an appreciable affinity for magnesium. A densely occupied monolayer acted in some degree, as an extension of the zeolite, and it was able to attract further cations from the solution phase (Gregg and Sing, 1982).
The adsorption phenomenon in zeolite ion-exchange isotherms was already seen (Shibata and Seff, 1997) indicating that XAZ values greater than 1 are possible to occur and the hypothesis of experimental errors should be completely discarded. It must be emphasized that all overexchanged values were neglected in the calculus of the selectivity coefficient of ion exchange and the thermodynamic properties. Concerning chromium exchange, it was observed in Fig. 1-d a pronounced convexly upward curvature of the Cr-NaX isotherms, which was a consequence of high interaction between Cr3+ ions and the negative framework charge. All Cr-NaX isotherms terminated at 82% of the theoretical exchange capacity, which means that chromium ions were not located in SI sites in the hexagonal prisms. Then, the steric hindrance experienced by the large Cr3+ ions in attempting to diffuse into the small cages and to exchange with the indigenous ions must be considered to explain the isotherm behavior. The respective Kielland plots presented non-linear shape for all cations and temperatures considered, which agrees with the results reported in Maes and Cremers (1975). The selectivity coefficients, calculated from the respective Kielland plots, were used to obtain the thermodynamic properties D Go and D Ho. These values are shown in Table 1. In this Table it was interesting to note important changes in D Go and D Ho values in the narrow temperature range of 30oC. It must be emphasized that the ion exchange mechanism is very temperature dependent and such feature was reflected in the thermodynamic process.
Large changes in thermodynamic properties were already seen in Cr-NaY ion exchange for the temperature range from 20 up to 60oC (Tagami et al., 2001). Besides the high temperature influence it may be supposed that multicomponent ion exchanges provide larger range of values of the thermodynamic properties due to the interaction of the in-going ions. In fact, as it will be discussed later, it happens for ternary isotherms. Considering the Ca-NaX and K-NaX systems, it can be observed in Table 1 that D Go values increased with increasing temperatures, which promoted less but still stable systems. This feature is in accordance with the shape of the isotherms. The enthalpy indicated that, for both cases, the ion exchange reactions were exothermic. As temperature increased, the reaction became more exothermic showing that the indigenous cations were more difficult to be removed in a reverse process. When the Mg-NaX system was considered, it was possible to observe that increasing temperatures provided more stable systems with a tendency to exothermic process. Comparing Ca-NaX and Mg-NaX systems there are pronounced differences in behavior of the metal ions related to the temperature influence. This fact is interesting because both cations have the same charge, nearly identical values of hydrated radii, that is 4.28 Å for Mg2+ and 4.12 Å for Ca2+ (Nightingale, 1959) – and not so different hydration energy of -377.9 kcal/g ion for Ca2+ and -452.7 kcal/g ion for Mg2+ according to Cotton and Wilkensen (1988). It seems that the coordination with framework oxygens and water molecules determine the selectivity in the zeolite system. The hydration characteristic of Cr3+ brought up interesting differences in DGo and DHo when compared to the other cations. Probably the chromium ions, at 30oC, were hexahydrated and mainly located in sites SII (Tagami et al., 2001), thus, no dehydration would be needed to exchange, providing an unstable system (D Go = 2.061 kJ/mol). Possibly, at this temperature, there was no sufficient energy to promote dehydration due to the high hydration energy of chromium ions ( = - 1005.5 kcal/g ion according to Cotton and Wilkensen, 1988). An increase in temperature provided more energy for the chromium ions to undergo dehydration and, as a consequence, Cr3+ ions were effectively exchanged in the large cages. When temperature increased to 60oC the system reached the minimum D Go values (-6.063 kJ/mol) indicating the maximum stability among all cations studied. Furthermore, Cr-NaX system became less endothermic when temperature increased because of the energy provided by temperature. It was also interesting to note that changes in temperature had little effect on the nature of the Cr-NaX isotherms.
= - 1005.5 kcal/g ion according to Cotton and Wilkensen, 1988). An increase in temperature provided more energy for the chromium ions to undergo dehydration and, as a consequence, Cr3+ ions were effectively exchanged in the large cages. When temperature increased to 60oC the system reached the minimum D Go values (-6.063 kJ/mol) indicating the maximum stability among all cations studied. Furthermore, Cr-NaX system became less endothermic when temperature increased because of the energy provided by temperature. It was also interesting to note that changes in temperature had little effect on the nature of the Cr-NaX isotherms.
Table 1: Thermodynamic properties of binary ion exchange
The almost superposed curves shown in Fig. 1-d indicated where the cations were located but no information related to the hydration of the exchanged cations could be provided. This fact explains this apparent contradictory phenomenon predicted by the isotherm and observed through the thermodynamic properties. According to the D Go results, it was possible to assure the NaX selectivity at 30oC was Ca2+ > K+ > Cr3+ > Mg2+, at 45oC was Ca2+ > Cr3+ > Mg2+ > K+ and at 60oC, Cr3+ > Mg2+ > Ca2+ > K+. These differences in the selectivity order are consequence of the hydration energy of each cation and its temperature sensitivity and ion-framework interaction.
The ternary isotherms were scanned following the same procedure described earlier. The ion exchange data are usually plotted in triangular figures but the exchange behavior is more clearly demonstrated considering XiZ plotted against XiS for each in-going ion i. The very interesting ternary isotherms are shown in Figs. 2 to 4. It may be observed that the multicomponent ion exchange behavior was totally different when compared to the binary isotherms as a result of competition towards the exchange sites of NaX. In competitive systems the interaction between the two in-going ions had also a huge influence ion exchange mechanism (Helferich, 1995) and as a general rule, XCrZ tended to be greater than XMZ, where M = Ca, K, or Mg. Fig. 2 shows the Cr/Ca isotherms. These isotherms had the closest XCrZ and XCaZ values at 30, 45 and even 60oC, indicating that, Cr3+ and Ca2+ cations might compete to the same type of sites. Therefore, in NaX, these results should be interpreted as implying that there was necessarily a direct competition between calcium and chromium for sites within the zeolites, mainly the ones easily accessible located in the supercages. The ternary systems Cr/K-NaX presented a high preference towards chromium as it is seen in Fig. 3-a, b and c. In all isotherms, there was a large difference between XCrZ and XKZ as a consequence of differences in charge and energy of hydration of each cation. The downward XKZ curvature occurred simultaneously with increasing XCrZ values. Since XKZ went through a maximum, mainly at 30oC and 60oC, Cr3+ ions must replace, not only Na+ ions, but also K+ ions during the course of exchange. These results suggested, firstly, that Cr3+ ions were much more preferred to K+ ions and secondly, when exchange was pushed towards the complete removal of Na+ ions from the zeolite phase, that the systems preferred Cr3+ ions located in sites already occupied by K+ ions. At 45oC some qualitative differences can be observed as a sigmoidal shape is found and some K+ acceptance occurred easier. Nevertheless, the K-NaX isotherm did not reach high values, which does not endanger previous discussions. The Cr/Mg ion exchanges shown in Fig. 4 were very similar in shape to Cr/K isotherms and all discussions related to that system could be applied. What is worth to note is that, in Cr/Mg-NaX isotherms, the difference between the magnesium and chromium isotherms was not so pronounced as it was in Cr/K-NaX systems; maybe because it was more difficult to exchange a divalent cation than a univalent one in a competitive system. The greatest difference occurred at 60oC where selectivity towards chromium was probably due to the loss of a great part of its hydration sphere. Concerning Cr/Mg-NaX isotherms, no adsorption phenomena occurred as it was observed in Mg-NaX systems, because no XMgZ values were greater than a unit. The presence of chromium in a competitive ion exchange process inhibited the adsorption of Mg2+ ions.
Representative Kielland plots for all ternary systems with pronounced non-linear shape for both in-going ions were obtained. With the thermodynamic equilibrium constants it was calculated the standard free energy and enthalpy changes of ion exchange, which are summarized in Table 2. It may be observed that all thermodynamic properties for multicomponent isotherms were very different if compared to binary ones, which emphasizes the influence of the interaction factor in the exchange mechanism. Concerning Cr/Ca systems (Table 2), it was observed that an increase in temperature from 30oC up to 60oC promoted a decrease in D Go values, which means more stability for both cations. At 45oC positive values of D Go were obtained, which suggested differences in stability due to interaction of the in-going ions. It was also possible to presume that competing for the same sites, the ion exchange process was influenced by the size-valence effect. Although trivalent cations are more preferable than divalent ones (Giannetto et al., 2000), the Ca2+ ion is less hydrated (Cotton and Wilkensen, 1988) and could penetrate more easily in the sodalite cages. Indeed, in all cases NaX seemed to be more selective towards calcium. As exchange proceeded at higher temperatures the Cr/Ca systems became endothermic exactly due to the necessity of losing its hydration sphere in order to reach less accessible sites.
a)

b)

c)

Fig. 2: Ternary exchange Cr/Ca-NaX isotherms at: a) 30 oC, b) 45 oC and c) 60oC. ( ) stands for Cr3+ ions and (O) stands for Ca2+ ions
) stands for Cr3+ ions and (O) stands for Ca2+ ions
a)

b)

c)

Fig. 3: Ternary exchange Cr/K-NaX isotherms at: a) 30 oC, b) 45 oC and c) 60oC. ( ) stands for Cr3+ ions and (
) stands for Cr3+ ions and ( ) stands for K+ ions
) stands for K+ ions
a)

b)

c)

Fig. 4: Ternary exchange Cr/Mg-NaX isotherms at: a) 30 oC, b) 45 oC and c) 60oC. ( ) stands for Cr3+ ions and (+) stands for Mg2+ ions
) stands for Cr3+ ions and (+) stands for Mg2+ ions
Concerning Cr/K systems, as temperature increased, the ion exchange process attained more stable energy levels. In all cases, the zeolite was more selective towards chromium than potassium, which was a consequence of in-going ion with different charges. In the case of Cr/Ca-NaX exchange on the contrary, as temperature increased, the systems became more exothermic, indicating that the interaction of the cations favored the exchange. The size-valence effect in Cr/Mg-NaX systems was as pronounced as in the Cr/Ca-NaX ones. At 30oC and 60oC the zeolite preferred Cr3+ instead of Mg2+ ions. However, at 45oC it was also interesting to note a preference towards magnesium although both exchanges were less favorable (D Go > 0) as it occurred with Cr/Ca-NaX. As temperature increased, the systems became more endothermic, showing that cations, mainly Cr3+, needed to lose their hydration sphere in order to be exchanged in the sites. The influence of the temperature, hydration and valence of the in-going ions and the interaction between them was reflected in the selectivity order based on the free energy values. Concerning the D Go presented in Table 2, it was possible to assure that the selectivity order was, at 30oC Ca2+ > Cr3+, Cr3+ » K+ and Cr3+ > Mg2+. At 45oC the sequence was Ca2+ > Cr3+, Cr3+ > K+ and Mg2+ > Cr3+ and finally at 60oC it was observed Ca2+ > Cr3+, Cr3+ > K+ and Cr3+ > Mg2+. In other words, the temperature of the system strongly influenced the multicomponent exchange.
Table 2. Thermodynamic properties of ternary ion exchange
IV. CONCLUSIONS
The data reported in this investigation fully confirmed the possibilities of practical applications of NaX for Ca2+, Mg2+, K+ and Cr3+ removal from single and multicomponent solutions as the zeolite displayed high selectivity to all cations studied. The temperature, the valence and hydration energy of the in-going ion influenced the ion exchange mechanisms and the thermodynamic properties. Moreover, different sites were involved in the ion-exchange processes. According to the D Go values, it was seen that the sequence of selectivity for binary isotherms is at 30oC Ca2+ > K+ > Cr3+ > Mg2+, at 45oC Ca2+ > Cr3+ > Mg2+ > K+ and at 60oC Cr3+ > Mg2+ > Ca2+ > K+. The difference in selectivity reflected distinct ion exchange mechanisms of each cation studied. The ternary isotherms were influenced by interaction between the in-going ions. As a result, the D Go and D Ho values were quantitatively very different when compared to binary isotherms. In multicomponent systems, NaX was more selective to Ca2+ instead of Cr3+ but the trivalent cation was more preferred than Mg2+ and had similar affinity with K+ ion at 30oC. The selectivity order at 45oC was changed again and the sequence was Ca2+ > Cr3+, Cr3+ > K+ and Mg2+ > Cr3+. At 60oC it was observed the sequence: Ca2+ > Cr3+, Cr3+ > K+ and Cr3+ > Mg2+. Based on the findings reported herein, it may be concluded that NaX did not have a pronounced selectivity towards chromium, in a binary ion exchange or even in multicomponent ones and more successful results may be obtained at 60oC.
REFERENCES
1. Barrer, R.M. and Klinowski, J., "Influence of Charge Density on Ion-exchange Properties of Zeolites." J. Chem. Soc., Faraday Trans. I., 68, 1956-63, (1972). [ Links ]
2. Cotton, F.A. and Wilkensen, G., Advanced Inorganic Chemistry, 5th ed., John Wiley & Sons, USA. (1988). [ Links ]
3. Giannetto, G., Montes, A. and Rodríguez, G., Zeolitas Características, Propiedades y Aplicaciones Industriales. Ed. Innovación Tecnológica. Venezuela (2000). [ Links ]
4. Gregg, S. J. and Sing, K. S. W., Adsorption, Surface Area and Porosity, Academic Press Inc., USA (1982). [ Links ]
5. Helferich, F., Ion Exchange, Dover Publications Inc., USA (1995). [ Links ]
6. Keane, M. A., "The Role of the Alkali Metal Co-Cation in the Ion Exchange of Y Zeolite IV. Cerium Ion Exchange Equilibria", Microporous Materials 7, 51-59. (1996). [ Links ]
7. Maes, A. and Cremers, A., "Ion Exchange of Synthethic Zeolite X and Y with Co+2, Ni+2, Cu+2 and Zn+2 Ions", J. Chem. Soc. Farad. Trans. I., 71, 265-274. (1975). [ Links ]
8. Meissner, H. P. and Kusik, C. L., "Activity Coefficients of Strong Electrolytes in Multicomponent Aqueous Solutions", AIChE J. 18, 2, 294-298. (1972). [ Links ]
9. Nightingale Jr., E.R., "Phenomenological Theory of Ion Solvation Effective Radii of Hydrated Ions", J. Phys. Chem., 63, 1381-1387 (1959). [ Links ]
10. Shibata, W. and Seff, K., "Pb2+ Exchange Isotherms for Zeolite Na-X at pH 5, 6, and 7", Zeolites, 19, 87-89. (1997). [ Links ]
11. Sherry, H., "The Ion-Exchange Properties of Zeolites. IV. Alkaline Earth Ion Exchange in Synthetic Zeolites Linde X and Y", The J. Phys. Chem., 72, 12, 4086-4094 (1968). [ Links ]
12. Tagami, L., Santos, O. A A., Sousa-Aguiar, E. F., Arroyo, P. A. and Barros, M. A. S. D., "NaY and CrY zeolites ion exchange. Thermodynamics", Acta Scientiarum, 23, 6, 1351-1357 (2001). [ Links ]
13. Zemaitis, Jr., J.F., Clark, D. M. and Rafal, M., Scrivner, N.C., Handbook of Aqueous Electrolyte Thermodynamics, DIPR, American Institute of Chemical Engineers, New York, USA (1986). [ Links ]














