Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Latin American applied research
versión impresa ISSN 0327-0793
Lat. Am. appl. res. v.34 n.1 Bahía Blanca ene./mar. 2004
Catalytic wet oxidation of ethanol in an integral trickle-bed reactor operated with liquid flow modulation
M. S. Fraguio, A. Muzen, O. Martínez1, P. Bonelli and M. Cassanello*
PINMATE-Dep. Industrias - FCEyN y Dep. de Tecnología Farmacéutica - Fac. de Farmacia y Bioquímica, Universidad de Buenos Aires. Int. Güiraldes 2620, 1428 Buenos Aires, Argentina. FAX:(+54-11)45763366.
miryan@di.fcen.uba.ar
1 Dep. de Ingeniería Química - Facultad de Ingeniería - Universidad Nacional de La Plata, Calle 47 esq. 1, 1900 La Plata, Argentina; CINDECA, Calle 47 No 257 - CC: 59, 1900 La Plata, Argentina.
ommartin@volta.ing.unlp.edu.ar
* Author to whom correspondence should be addressed
Abstract — The effect of liquid flow modulation on the performance of an integral trickle-bed reactor used for carrying out the catalytic wet oxidation of ethanol is analyzed. Particular emphasis is devoted to examine whether the induced periodic variations in conversion are affected by prolonged operation. A Pt/γ-Al2O3 catalyst (1% w/w, mean particle diameter dp = 0.003 m) is employed for the experiments. Liquid flow is open/closed periodically by means of a programmable logic controller with given splits and at different total cycle period. The reactor operates isothermally at 70oC during the whole cycles. The time dependence of the outlet ethanol concentration in the integral TBR is examined. In addition, the average conversion obtained with periodic operation is compared to that of the steady state at different cycle periods. It is found that the history of the operation mode has influence on the attained conversion, pointing to the existence of hysteresis affecting the particle wetting.
Keywords — Trickle-Bed Reactors. Liquid Flow Modulation. Periodic Operation. Ethanol Oxidation.
I. INTRODUCTION
Trickle-bed reactors (TBRs) are extensively used for many industrial applications, mainly in the refinery industry but also for new unconventional chemical and biochemical processes. Actual applications of these reactors have extended largely in various fields, including wastewater treatment, fine chemical, biochemical and electrochemical processes (Dudukovic et al., 1999). Among these new applications, the suitability of trickle-bed reactors for the catalytic wet oxidation (CWO) of organic compounds has been recently noticed (Pintar et al., 1997; Horowitz et al., 1999; Béziat et al., 1999; Fortuny et al., 1999). CWOs in three-phase reactors are particularly adequate for wastewater treatment when the concentration of organic compounds is too high or toxic to microorganisms (Matatov-Meytal and Sheintuch, 1998).
Traditionally, TBRs have been designed to operate under steady-state conditions. However, several recent studies have demonstrated that an unsteady-state (periodic) operation can yield better performance of these reactors, particularly for gas-limited reactions (Lange et al., 1994; Castellari and Haure, 1995; Khadilkar et al., 1999; Turco et al., 2001; Boelhouwer, 2001).
For CWO processes, the low solubility of oxygen in water may lead to a control of the gaseous reactant, specially for moderate to high temperature conditions (Tukac et al., 1999). Hence, high pressures are generally required. An alternative way to increase the accessibility of oxygen to the active sites arises from the possibility of having direct contact of the gaseous phase with the catalyst surface due to partial wetting of the catalysts. This can be achieved in trickle-bed reactors by low liquid flow rates or by using hydrophobic catalysts (Horowitz et al., 1999). Flow modulation of the liquid phase also leads to a lower wetting efficiency for the dry period. Then, the CWO in a TBR may be improved by periodic operation.
Therefore, in this work, the effect of liquid flow modulation on the performance of a TBR used for carrying out the CWO of ethanol is analyzed. A mini-pilot scale integral TBR packed with a Pt/γ-Al2O3 catalyst is employed for the experiments. Variations in the mean conversions obtained with periodic operation, in comparison with the attainable steady-state conversion, are determined for different modulation parameters. In addition, the time dependence of the outlet ethanol concentrations in the integral TBR is examined. Also, taking into account that only a partial oxidation of ethanol can be expected, intermediate products, like acetaldehyde and acetic acid, have been determined in the reactor effluent. This information will help to understand the influence of liquid flow modulation on the selectivity in a system of consecutive reactions.
II. EXPERIMENTAL
The catalytic wet oxidation of ethanol aqueous solutions was carried out at atmospheric pressure in a mini-pilot scale trickle-bed reactor. The reactor was made in acrylic, with 0.04 m internal diameter and 0.7m height. A schematic diagram of the experimental installation is presented in Fig. 1.
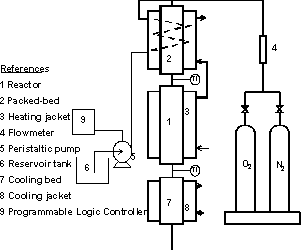
Fig. 1. Schematic diagram of the experimental installation
The reactor was externally heated with warm water in order to keep the temperature at 70oC during the whole cycles.
Aqueous ethanol solutions were contacted with O2 in a packed bed of glass beads placed before the reactor inlet to achieve thermal and vapor pressure equilibrium between the solutions and the gaseous phase. At the reactor outlet, another column filled with glass beads and provided with a cooling jacket allowed to absorb the non-reacted ethanol. Part of the generated acetaldehyde was also collected from the gas phase into the liquid and its concentration in the liquid phase could be determined. Temperature in the jacket was kept constant with flowing water.
Liquid flow was regulated with a peristaltic pump commanded by a programmable logic controller (PLC).
The catalyst employed was a conventional Pt/γ-Al2O3 catalyst (1% w/w), prepared by impregnation over γ-Al2O3 spheres of 0.003 m mean diameter.
The gaseous reactant was oxygen (99.9% v/v).
Ethanol concentrations in the feed solution were maintained at 0.006 or 0.03 kmol/m3 for all the experiments. Ethanol concentrations at the reactor inlet/outlet were determined with a Perkin Elmer Autosystem gas chromatograph using a FID detector. Acetic acid generated by reaction was determined by titration. Hence, the amount of generated gaseous products (gaseous acetaldehyde plus carbon dioxide) was estimated from the mass balance.
Gas-liquid equilibrium was tested by comparing the temperatures and liquid ethanol concentrations measured at the reactor inlet for several conditions with literature information (Hong et al., 1981). Good agreement was found.
Ethanol concentrations in the liquid feed and at the reactor outlet were used to calculate instantaneous ethanol conversions according to:
X = (NTi-NTo)/NTi (1)
NTi and NTo indicate ethanol moles at the inlet and outlet streams, respectively.
Before starting the experiments, the bed was flooded with the preheated liquid solution by closing the corresponding valves to ensure complete internal wetting of the catalyst and to heat the bed. Then, liquid and gas were circulated throughout the installation at high throughputs. Afterwards, the gas and liquid flow rates used for the experiments were fixed and the ethanol concentration at the reactor outlet was measured until steady state. The latter was identified by differences of less than 2% among ethanol concentrations for three consecutive samples. After steady state operation was attained, the liquid flow modulation was imposed. From that time on, liquid samples were taken periodically to follow the time dependence of the outlet ethanol concentration.
In addition, for different cycle periods, averaged final concentrations of ethanol and acetic acid were determined by collecting the liquid outlet stream for at least 40 minutes. Outlet streams were collected for periodic operations varying the cycle period from the smallest to the highest (forward exploration) and viceversa (backward exploration), leaving 20 minutes among different periods.
The mean liquid mass flow rate was fixed in 0.36 kg/m2s as a reference and will be called effective liquid mass flow rate, Lef. The cycle split, ratio of wet over total period, was set to 1/2 or 2/3. Therefore, the liquid mass flow rate was 2 Lef or 3/2 Lef during the wet period of the cycle, according to the cycle split imposed. There was no liquid flow during the dry period.
Experimental details and examined operating conditions are summarized in Table 1.
Table 1. Experimental details and examined operating conditions.

III. RESULTS AND DISCUSSION
The ratio of the average conversion obtained with liquid flow modulation, XFM, to the one obtained in steady state, XSS, is shown in Fig. 2 as a function of the cycle period, for two different splits. The attained conversion with periodic operation is determined by collecting the outlet stream during several cycles and that of the steady state corresponds to a liquid flow rate equivalent to the liquid flow rate during the wet period, multiplied by the split.


Fig. 2. Variations in the flow averaged conversions obtained with periodic operation in comparison with the attainable steady-state conversion for different cycle periods. (a) Ethanol concentration in the feed solution, Cf=0.03 kmol/m3; (b) Cf=0.006kmol/m3.
As observed in the figure, there is no clear evidence of a persistent improvement in reactor performance due to the periodic operation, particularly for the larger ethanol concentration (Fig. 2a). The overall attained conversion remains always within ± 20% of the steady state conversion.
For the lower ethanol concentration, a neater trend is observed (Fig. 2b). Reactor performance is slightly improved for high frequency modulation and a marked detriment is observed for long cycle periods, which is likely arising from a lack of liquid reactant since the detrimental trend is more evident for the lower split.
The variations observed with respect to the steady state behavior can be attributed only to hydrodynamic effects, mainly the combined effect of an overall reduction of the mean wetting efficiency and shortage of the liquid reactant, since the experiments were carried out under isothermal conditions.
For a gas-limited reaction, like ethanol oxidation, a decrease in wetting efficiency can improve the supply of O2 to the particle, increasing the reaction rate and the conversion (Horowitz et al., 1999). A gas-limited reaction can be defined using the criterion proposed by Khadilkar et al. (1999). For our system at inlet conditions, the index proposed by Khadilkar et al. is higher than 10, which means clearly a gas-limited reaction. But, when the ethanol conversion is high, particularly during the dry period, the index diminishes to values between 1 and 2 and the reaction can turn to be liquid-limited. Therefore, during the dry period, the reaction rate can increase at the start due to a lower wetting efficiency and then decrease if the dry period is long enough due to a deficit in the liquid reactant.
Considering the previous argument, even if the reaction is gas-limited at inlet conditions, an improvement in reactor performance is conditioned by the possibility of a shortage in the liquid reactant for prolonged cycle periods.
The influence of the liquid flow modulation on the instantaneous ethanol conversion is illustrated in Fig. 3 for a cycle period of 900s, a split of 2/3 and an ethanol concentration in the feed solution of 0.006 kmol/m3. The conversions attained in the steady state operation with the effective liquid mass flow rate (SSef : L = Lef) and with the liquid mass flow rate used for the wet period (SS : L = 3/2Lef) are indicated in the figure for comparison.
The instantaneous conversion presents an almost periodic behavior, ranging from the conversion for steady state with the liquid flow rate of the wet period to a quite larger conversion, around 90%. The higher values are attained in the first instants of the wet period of the cycle. Then, the conversion decreases tending to the value obtained for steady state. In the dry period, the conversion starts to increase again.
Superimposed to the almost periodic behavior, an overall slow decreasing trend can also be appreciated. This slow but persistent variation suggests that the mean wetting efficiency of the catalyst particles may depend on the past history of the liquid flow rate. A long cycling operation with relatively large cycle periods may induce variations in the wetted zones of the particles, even if the mean wetting efficiency remains constant. Different liquid pathways may appear while starting each new wet period of the cycle. This effect may be less apparent for short cycling periods since the liquid may not have drained completely from the column when the new wet period starts.

Fig. 3. Time dependence of the instantaneous ethanol conversion for a total cycle period of 900 s. Split=2/3; Cf=0.006kmol/m3. Open and close periods indicated in the figure as a solid line in the bottom.
As already mentioned, another possible influence of a prolonged cycling operation with long cycle periods could be a progressive decrease of the liquid reactant concentration within the catalyst. This effect should appear if the reactant is almost completely consumed during the dry period and the wet period is not long enough to reach the liquid reactant concentration of the steady state conditions.
To examine the hypothesis that the conversion is affected by the past history of the liquid flow rate, the cycle period was varied either by increasing it from the lowest values to the highest (forward exploration) or viceversa (backward exploration). Measured ethanol conversions are represented vs cycle period in Fig. 4 for both exploration routines and two different splits.
As observed in the figures, larger ethanol conversions were attained when the cycle period was decreased progressively from the largest value (900s) to the lowest, whatever the split used.
These results might be related to a slight variation in the mean overall wetting efficiency. A decrease in the mean wetting efficiency affects in two opposite ways. On one side, it favors the access of the gaseous reactant (oxygen) into the particles, which improves the reaction rate. On the other side, it hinders the accessibility of the liquid reactant that may be in equilibrium at the operation start up, but it is highly consumed during the dry period and may not be readily reposed during the wet period. In addition, if the wetted regions are better distributed, the effectiveness factor will be higher.


Fig. 4. Variations in the flow averaged conversions obtained with liquid flow modulation for different cycle periods and starting the operation either from the longest cycle period (backward exploration) or viceversa (forward operation): a) split = 2/3, b) split = 1/2. Cf = 0.03 kmol/m3.
When the experiments are started with a long cycle period, the initial condition for this period is that of equilibrium for the alcohol concentration and, since the reactor was previously flooded, wetted zones may distribute homogeneously, covering the particles. No preferential paths have still imposed. This fact, added to a probable slightly lower mean wetting efficiency that may characterize longer cycles, leads to a higher conversion. A decrease in the cycle period implies shorter dry periods and the liquid reactant concentration may not be consumed largely, and thus is able to be reestablished during the wet period. Then, only the effect of a lower mean wetting efficiency would remain.
A continuously cycling operation from the shorter to the higher cycle periods may only reflect the effect of an apparently higher mean wetting efficiency that always leads to lower conversions. The lack of liquid reactant would have effect only at the last experiments (long cycle periods).
The influence of the past history is more apparent for the lower split, which supports the previous discussion.
Finally, the influence of the flow modulation on the product distribution was tentatively examined. The selectivity towards formed products, calculated as the obtained concentration referred to the ethanol consumption, is represented as a function of the cycle period in Fig. 5. "Gaseous products" refer to the carbon dioxide plus the acetaldehyde, which is present both in the liquid and gas phase phase.

Fig. 5. Variations in the selectivity towards formed products obtained with liquid flow modulation for different cycle periods and splits. Cf=0.03kmol/m3
The selectivity towards the generation of gaseous products is remarkably larger than for the formation of the acetic acid. Considering the refractory character of the low-molecular weight carboxylic acids (Beziat et al., 1999; Kolaczkowski et al., 1999; Klinghoffer et al., 1998), it could be convenient to prevent their formation. The intermediate product (acetaldehyde), present in large amount as evidenced by its concentration in the liquid, is mostly in the gaseous phase due to its high vapor pressure and could be directly absorbed into an appropriate solvent and reused.
The selectivity towards acetic acid is slightly increased by liquid flow modulation compared to that obtained in steady state conditions. The largest effect appears at long cycle periods and for the lower split, for which reactor performance is also worse. This could arise from a lack of ethanol to oxidize during the dry period plus a larger oxygen concentration that may improve the oxidation of acetaldehyde to the acid.
IV. CONCLUSIONS
The effect of using liquid flow modulation for the catalytic wet oxidation of ethanol in an isothermal mini-pilot trickle-bed reactor was examined. The following trends were found within the explored conditions:
- Larger conversions are attained for shorter cycling periods, particularly for smaller splits. This result was more evident for the lower ethanol concentration in the feed solution.
- The time variation of the ethanol concentration leaving the reactor at the examined condition was almost periodic. Smallest values were obtained at the start-up of the wetted period. Superimposed to the almost periodic behavior, a slight negative trend in ethanol conversion was also appreciated as time evolves.
- The past history of the liquid flow apparently has an influence on the attainable ethanol conversion. Hence, the overall procedure used to make the experiments should be carefully systematized.
- The product distribution is affected by liquid flow modulation. Longer dry periods seem to have a positive effect on the selectivity towards acetic acid formation.
In general, the influence of liquid flow modulation on product distribution and the quasiperiodic variation of the outlet concentrations suggests that, even if no variation is obtained in reactor performance, the use of periodic operation can be beneficial to direct the selectivity towards the formation of a specified product or to separate streams with different conversion levels.
NOMENCLATURE
| Cf | Ethanol concentration in the feed solution, kmol/m3 |
| G | gas mass flow rate, kg/s.m2 |
| L | liquid mass flow rate, kg/s.m2 |
| Lef | effective liquid mass flow rate, kg/s.m2 |
| NTi | total number of moles entering the system, including gas and liquid phases, kmol |
| NTo | total number of moles leaving the system, including gas and liquid phases, kmol |
| X | ethanol conversion |
| Subscripts | |
| FM | periodic operation |
| SS | stationary state |
ACKNOWLEDGMENTS
Financial support from Universidad de Buenos Aires (UBA) and Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina, is gratefully acknowledged.
REFERENCES
1. Boelhouwer, J.G. Nonsteady operation of trickle-bed reactors: Hydrodynamics, mass and heat transfer, PhD Thesis, Technische Universiteit Eindhoven, The Netherlands (2001). [ Links ]
2. Béziat, J.C., Besson, M., Gallezot, P., Durécu, D. "Catalytic Wet Air Oxidation on a Ru/TiO2 Catalyst in a Trickle-Bed Reactor", Ind. Engng. Chem. Res. 38, 1310 (1999). [ Links ]
3. Castellari, A., Haure, P.M. "Experimental study of the periodic operation of a trickle-bed reactor", AIChE J. 41, 1593 (1995). [ Links ]
4. Dudukovic, M.P., Larachi, F., Mills, P.L. "Multiphase reactors - revisited", Chem. Eng. Science 54, 1975 (1999). [ Links ]
5. Fortuny, A., Bengoa, C., Font, J., Castells, F., Fabregat, A. "Water Pollution Abatement by Catalytic Wet Air Oxidation in a Trickle-Bed Reactor", Catalysis Today 53, 107 (1999). [ Links ]
6. Hong, J., Ladisch, M.R., Tsao, G.T. "Vapor-Liquid Equilibria of the Water-Ethanol systemat Low Alcohol Concentrations", J. Chem. Eng. Data 26, 305 (1981). [ Links ]
7. Horowitz, G.I., Martínez, O.M., Cukierman, A.L., Cassanello, M.C. "Effect of the catalyst wettability on the performance of a trickle-bed reactor for ethanol oxidation as a case study", Chem. Eng. Science 54, 4811 (1999). [ Links ]
8. Khadilkar, M.R., Al-Dahhan, M.H., Dudukovic, M.P. "Parametric study of unsteady-state flow modulation in trickle-bed reactors", Chem. Eng. Science 54, 2585 (1999). [ Links ]
9. Klinghoffer, A.A., Cerro, R.L., Abraham, M.A. "Catalytic wet oxidation of acetic acid using platinum on alumina monolith catalyst", Catalysis Today, 40, 59 (1998). [ Links ]
10. Kolaczkowski, S.T., Plucinski, P., Beltran, F.J., Rivas, F.J., McLurgh, D.B. "Wet air oxidation: a review of process technologies and aspects in reactor design", Chem. Eng. J., 73, 143 (1999). [ Links ]
11. Lange, R., Hanika, J., Stradiotto, D., Hudgins, R.R., Sylveston, P.L. "Investigation of periodically operated trickle-bed reactors", Chem. Eng. Science 49, 5615 (1994). [ Links ]
12. Matatov-Meytal, Y., Sheintuch, M. "Catalytic abatement of water pollutants", Ind. Engng. Chem. Res. 37, 309 (1998). [ Links ]
13. Pintar, A., Bercic, G., Levec, J. "Catalytic liquid-phase oxidation of aqueous phenol solutions in a trickle-bed reactor", Chem. Eng. Science 52, 4143 (1997). [ Links ]
14. Tukac, V. Vokál, J., Hanika, J. "Mass-Transfer-Limited Wet Oxidation of Phenol", Chem. Papers 53, 357 (1999). [ Links ]
15. Turco, F., Hudgins, R.R., Silveston, P.L., Sicardi, S., Manna, L., Banchero, M. "Investigation of periodic operation of a trickle-bed reactor", Chem. Eng. Science 56, 1429 (2001). [ Links ]
Received: January 17, 2002.
Accepted for publication: May 30, 2002.
Recommended by Subject Editor Ana Lea Cukierman.














