Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Latin American applied research
versión impresa ISSN 0327-0793
Lat. Am. appl. res. v.34 n.1 Bahía Blanca ene./mar. 2004
Dryout phenomenon in the periodic operation of a trickle bed reactor
J. Ochoa2, J. Cechini1 and P. Haure1,*
1 INTEMA, CONICET, Facultad de Ingeniería, UNMdP, Av. J.B. Justo 4302, 7600 Mar del Plata, Argentina.
phaure@fi.mdp.edu.ar; jorce@fi.mdp.edu.ar
2 PROIMI, CONICET, Avda. Belgrano y Pje. Caseros, 4000 S.M. de Tucumán, Argentina.
jochoa@proimi.edu.ar
* To whom correspondence should be addressed.
Abstract — Periodic operation was investigated for the hydrogenation of alpha-methylstyrene to cumene over 0.5% Pd on alumina spheres. The effect of several variables such as gas flow rate, gas and liquid composition, and liquid feed temperature on unsteady-state liquid flow modulation in a Trickle Bed Reactor is studied. These parameters affect the depletion time of the volatile reactant as well as the temperature profiles of the dry cycle. A criterion is presented that allows for an estimation of the maximum temperature during the dry cycle based on mass and energy balances.
Keywords — Trickle Bed Reactor. Periodic Operation. Gas-Phase Reaction. Dryout.
I. INTRODUCTION
Previous work on the periodic operation of a Trickle Bed Reactor (TBR) has demonstrated that for the hydrogenation of alpha methyl styrene (AMS) to cumene on Pd/Al2O3 catalyst, reaction rates are increased up to 400% with respect to the steady state results (Castellari and Haure, 1995). In this mode of operation, circulation of the liquid phase is switched on and off while the gas passes continuously through the reactor. Silveston et al. (1995) pointed out that due to the complexity of the unsteady operation and the probability of higher costs than the conventional procedure, cycling is recommended if the rate enhancement is at least higher than 100%.
The performance of a TBR under cycling is extremely complex, especially in the case of the gas-limited, liquid volatile, exothermic reaction situation. The performance depends on the switching between wet and dry operation (Gabarain et al., 1997). In the non-wet cycles, wetting of the packing is incomplete. The reaction rate can be greater or smaller than the rate observed over completely wetted packing. This depends on whether the limiting reactant is present only in the liquid phase or in both the gas and liquid phases. If the reaction is gas limited, rates will be higher because the gas reactant can access the catalyst pores from the externally dry area. When the reaction is liquid limited and the liquid reactant is non-volatile, a decrease in the wetting efficiency will cause a decrease in the reaction rate. But if the liquid reactant is volatile and significant heat effects are also present, then, a gas phase reaction can occur on the dry catalyst resulting in higher rates and temperatures (Al-Dahhan and Dudukovic, 1995). The associated increase in the local reaction rate and the temperature rise may be a feature of concern because it can lead to the formation of uncontrolled hot-spots and the subsequent sintering of the catalyst, runaway conditions and undesirable side reactions, as pointed out by Hanika et al. (1975). Proper selection of the cycling variables is strongly recommended to exploit the vaporization phenomena while avoiding related problems.
The occurrence under steady-state conditions of vaporization during reaction in multiphase reactors at the single pellet level is well documented (Hanika et al., 1976; Watson and Harold, 1993, 1994). In particular, Watson and Harold (1993) studied the Pd-catalyzed hydrogenation of AMS (to cumene) and of cyclohexene (to cyclohexane) in a single pellet catalytic reactor. The key difference between the two reactions is the volatility of the less volatile reactant. The experimental data reveal interplay between the exothermic reaction, the endothermic vaporization and internal and external transport processes. For the case of drying with hydrogenation, they observed a departure from traditional drying theory, especially in the initial period due to the influence of the latent heat of vaporization, the heat conduction and the heat generated by the exothermic reaction. In general, hydrogenation accelerates the drying process because the reaction on dewetted sites speeds up the dryout phenomenon. The rate of drying depends on the volatility of the liquid and, if sufficient time elapses such that a fraction of the catalyst becomes exposed to the gas, a much more rapid gas-phase catalytic reaction occurs with an accompanying temperature excursion.
Gabarain et al. (1997) have proposed a phenomenological model that explains the events associated with periodic interruption of the liquid phase to a TBR. Experimental and model results compare reasonably well. Cycling deliberately creates hot spots during the "dry" cycles due to the onset of gas-phase reaction and results in higher conversions compared to the conventional operation. The overall reaction rate is an average between the diffusion-controlled liquid phase reaction and the more rapid gas-phase catalytic route. The reactor operates at higher average temperatures compared to the steady state. However, the phase transition is responsible for the large magnitude of the rate increase. The extent of pore filling depends on several variables, such as catalytic activity, support permeability, heat of reaction, volatility of the liquid components, overall reaction energies, gas flow rate and composition, etc (Watson and Harold, 1994). Once the gas phase reaction begins, the AMS is rapidly consumed. The optimum conditions for cycling are a strong function of the "depletion time" (td) of the liquid reactant and can be achieved by a proper selection of splits and periods providing that all other conditions remain constant.
Recent studies of the effect of periodic operation on TBR performance include those of Lange et al. (1999)) and Khadilkar et al. (1999). In both studies AMS was hydrogenated to cumene. Lange et al. (1999) have reported that under liquid flow interruption the time-average conversion is higher than under steady-state operating condition, depending on the choice of the operating parameters. A dynamic heterogeneous mathematical model simulated experimental trends although the conversions predicted were significantly higher than the experimental data. Khadilkar et al. (1999) investigated the reaction system under gas and liquid-limited conditions. Performance enhancement under gas-limited conditions (with ON-OFF flow modulations) was observed to be greater when gas supply is enhanced. Performance enhancement under liquid-limited conditions was observed only with BASE-PEAK flow modulation .
Stradiotto et al. (1999) studied the hydrogenation of crotonaldehyde under steady state and periodic operation, for liquid limited conditions. The liquid reactants were too dilute to show significant exothermicity, and they observed a 50% increase in the crotonaldehyde consumption rate for lower liquid flow rates.
The above mentioned works were conducted under conditions distant from the semi-runaway situation studied by Castellari and Haure (1995).
In the past, phase transition was considered a dangerous issue, but, if properly controlled, this Process Intensification technology can advantage conventional methods. The use of a controlled phase transition as a way to benefit reactor performance could result in advantages in process efficiency, energy and investment savings.
The objective of this work is to experimentally examine the effect of several variables such as gas flow rate, gas and liquid composition and liquid feed temperature on the dryout phenomenon observed during the periodic operation of a TBR. The above-mentioned parameters influence the degree of external wetting, liquid holdup and transport mechanisms. The couplings between the extent of pore emptying, partial external wetting, transport and reaction can be assessed by means of the model previously developed by Gabarain et al. (1997). The maximum temperature attainable during cycling is predicted from mass and energy balances in the dry cycle.
II. EXPERIMENTAL DETAILS
The hydrogenation of AMS to cumene has been extensively studied by others (Morita and Smith, 1978; Herzkowitz et al., 1979; Cini and Harold, 1991). Reaction rate is rather fast under mild conditions and cumene is the only measurable product. Properties of the system are shown in Table 1.
Table 1. System Properties

Properties of the catalyst and operating and analysis procedures are offered in detail by Castellari and Haure (1995). Bed characteristics are given in Table 2.
Table 2. Bed Characteristics

A sheathed thermocouple was inserted axially in the middle of the catalytic bed. A data acquisition device recorded local bed temperatures continuausly. A three-way solenoid valve (Jefferson model 365) activated by timers was used to generate liquid flow rate variations. Hydrogen was saturated with AMS before each run. Experiments were performed randomly, alternating different cycling runs with steady state measurements to check for catalyst activity. No deactivation of the catalyst was observed. Reproducibility was good. The operating conditions were chosen based in our previous experimental results (Castellari and Haure (1995), Gabarain et al. (1997)). The selected values allowed us to study the impact of the desired variables in our experimental setup. Experimental conditions used in most of the runs are listed in Table 3.
Table 3. Operating Conditions

Liquid samples were taken a minute before ending each "wet cycle" and analyzed in a GC (Hewlett-Packard 5890 A). Changes in the cumene concentration with time were used to evaluate global reaction rates following the procedure described by Castellari and Haure (1995). Reaction rates are expressed in mol of cumene / s gr. of catalyst.
III. RESULTS AND DISCUSSION
The behavior of the catalytic bed during periodic flow interruption can be explained by means of a simple phenomenological model (Gabarain et al., 1997). Consider a catalytic pellet, as shown in Fig. 1. During the wet cycle (Zone A) the pellet is completely wet, the dissolved gas species is the limiting reactant and the overall rate is external mass transfer controlled, as in conventional TBR operation. Once the liquid flow is halted, the bed partially drains and the catalytic reaction proceeds between the flowing hydrogen and the liquid holdup. The flowing gas does not easily dissipate heat generated. Evaporation of the liquid phase, followed by a much rapid gas-solid catalyzed reaction, may occur in Zone B1. The overall reaction rate has then two contributions: one from the wetted areas or liquid-solid catalyzed reaction and the other from the non-wetted surfaces. The static holdup diminishes with time, due to evaporation. Once the external holdup is depleted, the reaction takes place between the evaporated internal holdup and the gas reactant (Zone B2). Here we assume that the reaction takes place via gas-solid catalysis. When the dry out is completed, or depletion time (td), the bed temperature decreases (Zone C).
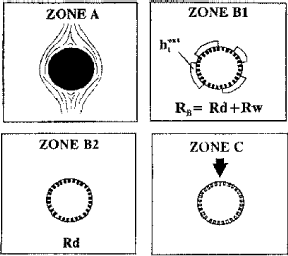
Figure 1. Behavior during periodic operation
Figure 2 shows a typical temperature profile for period 40 min and split 0.5. Three different zones can be distinguished, corresponding to the events shown in Figure 1.
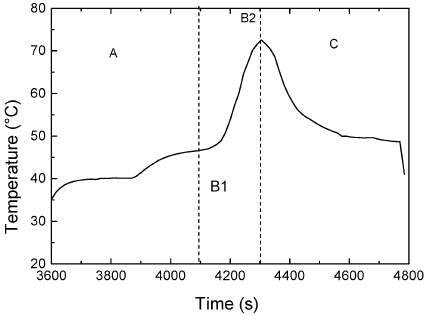
Figure 2. Typical temperature profile. Split 0.5; Period 40 min.
Experiments were carried out varying liquid feed temperature, gas flow rate and gas and liquid composition. These variables influence the drying rate of the liquid film, approximated by the transport of AMS through a boundary layer:
Rd = kg ( Pv (Ts) -Pb) (1)
where Pb is the partial pressure related to the bulk. The gas-solid mass transfer coefficient kg was evaluated according to Goto and Smith (1975).
Based on mass and energy balances in the dry cycle, the following relationship allows evaluating the maximum temperature (Tmax) as a function of the initial temperature (T0) liquid vapor pressure (Pv), reaction and vaporization heats (λ r and λ v), particle diameter (dp) bed porosity (ε s), solid density (ρ s) and activation energy (Ea):
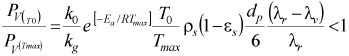 | (2) |
The kinetic constant k0 was obtained following Cini and Harold (1991).
A. LIQUID FEED TEMPERATURE
Castellari and Haure (1995) have found that liquid feed temperature does not have an important influence at steady state conditions where mass transfer resistances are significant. However, it is an important variable while operating a TBR in circumstances where vaporization and gas-solid catalyzed reaction are possible.

Figure 3. Temperature profiles at different liquid feed temperatures. Gas Flow rate 900 cc/min. Split =0.5. Period = 20 min. Ccummene < 15%.
Figure 3 shows the temperature profiles obtained at three different liquid feed temperatures and gas flow rate 1500 cc/min. Table 4 compares experimental and theoretical results obtained by the model of Gabarain et al (1997).
Table 4. Comparison between experimental and theoretical results for different liquid feed temperature. Gas flow rate 1500 cc/min; split 0.5 and period 20 min. Ccumene < 15%.
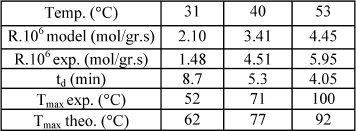
As liquid feed temperature was increased, maximum temperatures reached were higher and a displacement between the maximum is found. Profiles have approximately the same shape, indicating the occurrence of similar phenomena. The behavior can be represented by the events mentioned in Section III. Temperature raise enhances the reaction rate, as expected. The liquid vapor pressure is higher, increasing the drying rate according to Eq. (1). It also allows the gas phase catalytic reaction to start earlier and accelerates the pore emptying process, modifying the depletion time.
B. GAS FLOW RATE
Temperature profiles for three different flow rates of pure hydrogen (400, 900 and 1500 cc/min) are plotted in Fig. 4. The maximum temperature rise and the depletion time varied. The gas flow rate influences the mass transfer coefficient in Eq. (1), and also the heat transfer coefficient. Mass transfer and heat removals are enhanced and they have an opposite effect on reaction rate.
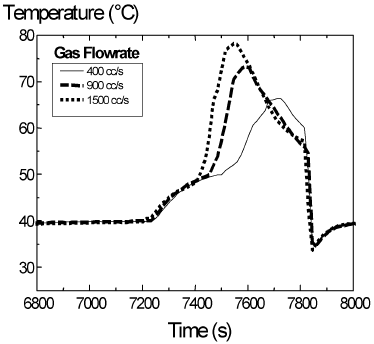
Figure 4. Temperature profiles for different gas flow rates. Liquid feed temperature = 41o C. Split = 0.5 and period = 20 min. Ccumene < 15%.
The more rapid initial drying obtained with the higher hydrogen flow rate resulted in a more rapid temperature rise and vaporization of liquid followed by pore emptying. Watson and Harold (1993) reported similar results. However, the heat transfer coefficient is also enhanced, allowing the bed to remove heat more efficiently. Maximum temperatures differ in less than 20o C. A comparison of predicted and experimental results is shown in Table 5.
Table 5. Comparison between experimental and theoretical results for different gas flow rates. Temperature 41o C; split 0.5 and Period 20 min. Ccumene < 15%.
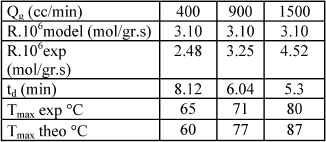
The model does not predict accurately the variations in the experimental rate results probably due to the calculated heat transfer coefficients. However, maximum temperatures are estimated reasonably well by Eq. (2).
C. GAS COMPOSITION
The hydrogen concentration (CH2) in the gas feed was varied. Mixtures of high purity hydrogen and nitrogen were fed continuously through the reactor.Temperature profiles in Fig. 5 reveal that the maximum temperature reached is the same, but there is a shift in the depletion time. A maximum at CH2 25% is found after 25 min (not shown). lf CH2 is below a critical value, temperature remains constant. This can be explained as follows: once the gas phase reaction starts, it just depends on gas phase AMS concentration, given by the holdup, provided that there is enough hydrogen to sustain the zero order dependence. Although H2 is not pure, its concentration is high enough to be considered in excess in the gas phase step. As hydrogen concentration becomes lower, mass transfer of hydrogen becomes limiting.
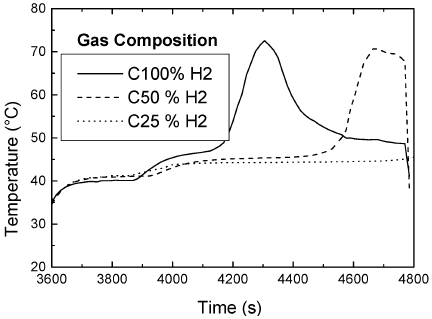
Figure 5. Temperature profiles for different hydrogen concentrations. Liquid feed temperature = 41 o C, gas flow rate 1500 cc/s, split = 0.5 and period = 20 min. Ccumene < 15%.
Experimental rates were also monitored. In general, higher experimental rates correlated positively with higher fractions of H2 in the gas feed. Below a critical value of hydrogen no reaction is observed. When yH2 is higher than 25% maximum temperatures reached are identical. Theoretical and experimental rates are in agreement as shown in Table 6.
Table 6. Comparison between experimental and theoretical results. Temperature 41 o C; split 0.5 and period 20 min. Ccumene < 15%.
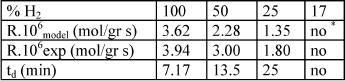
* not observed
Performance enhancement under gas-limited conditions with ON-OFF cycling was observed to be greater when gas supply is enhanced. Results are in agreement with Khadilkar et al. (1999).
D. LIQUID CONCENTRATION
The effect of the concentration of the liquid reactant was also studied. The temperature profiles in Fig. 6 show that a non-pure liquid reactant feed reaches lower temperatures during the dry cycle. Overall reaction rates are 4.52.10-6 and 2.53.10 -6 mol/gr.s for cumene concentration lower than and higher than 15 %. The dilution of the liquid reactant does not affect the results obtained during the wet cycle, where reaction is controlled by the transport of hydrogen, but does modify behavior during the dry cycle in which reaction depends on gas phase AMS concentration, provided that there is enough hydrogen. It seems obvious that if the liquid reactant is very diluted (as in liquid-limited reactions), the benefits from ON-OFF cycling will be reduced
Khadilkar et al. (1999) investigated the effect of liquid reactant feed concentration under gas-limited conditions by evaluating enhancement at two different concentrations, at different cycle splits and different liquid mass velocities. They observed higher enhancement at lower liquid feed concentrations. Since their experiments were performed at no semi-runaway conditions, comparison with our results is useless. More experiments are necessary to investigate this effect.

Figure 6. Temperature profiles for different liquid feed concentrations. Liquid feed temperature = 41 o C, gas flow rate 1500 cc/s, investigate this effect.
E. EXTENSION OF THE CRITERION
The criterion in Eq. (2) could be used to estimate maximum experimental temperature found by others. Table 7 compares results for different hydrogenation systems.
Table 7. Operating conditions used in Eq. (2)
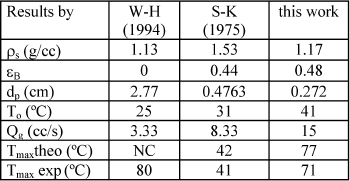
NC: iteration procedure did not converge.
Sedricks and Kenney (S-K) (1975) studied steady state hydrogenation of crotonaldheyde (CA). A hot spot was encountered, working at low liquid flow rates, and the probability of the existence of gas-solid catalytic reaction was mentioned. Watson and Harold (W-H) (1994) reported a temperature rise of 55 oC when a single catalytic pellet, cyclohexene (CH) wetted, was exposed to a pure hydrogen gas stream. Finally, our results for the hydrogenation of AMS are also reported. Physical and chemical properties for the AMS, CH and CA are detailed in Table 8. Watson and Harold (1994) results on a single pellet could not be reproduced because the iteration procedure did not converge.
Table 8. Physical and chemical properties
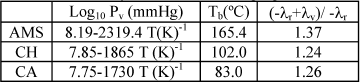
Maximum temperatures predicted by Eq. (2) are generally higher than experimental values. The assumption that the gas-solid catalytic reaction is taking place in all the catalyst plus the uncertainty in estimating parameters are the explanation for the differences between experimental and theoretical values. However, the criterion permits an assessment of the impact of several variables on reactor performance.
IV. CONCLUSIONS
Periodic operation of TBR deliberately modifies the wetting condition of the catalytic bed, allowing creating dry areas. If properly controlled, this can improve reactor performance, especially in gas-limited - liquid volatile reactions with important heat effects. In the dry cycles, reaction proceeds between the liquid holdup (internal and external) and the flowing gas. The liquid holdup diminishes with time, until it is fully depleted (dryout condition). This allows to operate the reactor under semi-runaway conditions. The gas flow rate, compositions of the gas and liquid feed, and temperature have an important effect on reactor performance during cycling. A criterion is presented that allows for an estimation of the hot spot or maximum temperature during the dry cycle. Theoretical temperatures are, in general, higher than experimental values because it is assumed that the gas-solid catalyzed reaction is taking place in all the catalyst. The criterion could be used to predict maximum temperatures during dryout phenomenon in similar reaction systems in which reactants are present in both phases and the system is exothermic.
ACKNOWLEDGMENTS
We want to express our gratitude to Mr Héctor Asencio and Ms Carmen Rodríguez and Eng. M.A. Ayude for their valuable assistance. We are deeply indebted to Professor Jorge F. González whose discerning comments improved the readability of the article.
REFERENCES
1. Al-Dahhan M.H. and M. Dudukovic. "Catalyst Wetting Efficiency in Trickle Bed Reactors at High Pressure". Chem. Eng. Sci. 50, 15, 2377-2389 (1995). [ Links ]
2. Castellari A.T. and P.M. Haure. "Experimental Study of the Periodic Operation of a Trickle Bed Reactor". AIChE J. 41, 6, 1593-1597 (1995). [ Links ]
3. Cini P. and M. P. Harold. "Experimental Study of the Tubular Multiphase Reactor". AIChE J. 37, 7, 997-1008 (1991). [ Links ]
4. Gabarain L., A. T. Castellari, J. Cechini, A. Tobolski and P. Haure. "Analysis of Rate Enhancement in a Periodically Operated Trickle Bed Reactor". AIChE J. 43, 1, 166- 172 (1997). [ Links ]
5. Goto, S. and J.M. Smith, "Trickle Bed Reactor Performance: 1. Hold-up and Mass Transfer Effects", AIChE J. 21, 6, 706-713 (1975). [ Links ]
6. Hanika J., K. Sporka, V. Ruzicka and J. Krausova, "Qualitative Observations of Heat and Mass Transfer Effects on the Behavior of a Trickle Bed Reactor", Chem. Eng. Comm. 2, 19-26 (1975). [ Links ]
7. Hanika J., K. Sporka, V. Ruzicka and J.H. Rstka "Measurement of Axial Temperature Profiles in an Adiabatic Trickle Bed Reactor". Chem. Eng. J. 12, 193-205 (1976). [ Links ]
8. Herzkowitz, M., R.G. Carbonell and J.M. Smith. "Effectiveness Factors and Mass Transfer in Trickle Bed Reactors". AIChE J. 25, 272 -282 (1979). [ Links ]
9. Khadilkhar, M.R., P.L. Mills and M.P. Dudukovic "Trickle Bed Reactor Models for Systems with a Volatile Liquid Phase". Chem. Eng. Sci. 54, 2421-2431 (1999). [ Links ]
10. Lange R., R. Gutsche and J. Hanika. "Forced periodic Operation of a Trickle Bed Reactor". Chem. Eng. Sci. 54, 2569-2573 (1999). [ Links ]
11. Morita, S. and J.M. Smith "Mass Transfer Limitations in a Trickle Bed Reactor" I&E Fund. 1, 113-123 (1978). [ Links ]
12. Sedricks, W. and C.N. Kenney, "Partial wetting in Trickle Bed Reactors - the reduction of crotonaldehyde over a palladium catalyst". Chem. Eng. Sci. 28, 559-570 (1975). [ Links ]
13. Silveston P.L., R.R. Hudgins and A. Renken. "Periodic Operation of Catalytic Reactors - Introduction and Overview". Catalysis Today 25, 91-112 (1995). [ Links ]
14. Stradiotto D.A., R.R. Hudgins and P.L. Silveston. "Hydrogenation of Crotonaldehyde under Periodic Flow Interruption in a Trickle Bed". Chem. Eng. Sci. 54, 2561-2568 (1999). [ Links ]
15. Watson P.C. and M.P. Harold. "Dynamics Effects of Vaporization with Exothermic Reaction in a Porous Catalytic Pellet". AIChE J. 39, 6, 989-1013 (1993). [ Links ]
16. Watson P.C. and M.P. Harold. "Rate Enhancement and Multiplicity in a Partially Wetted and Filled Pellet: Experimental Study". AIChE J. 40, 1, 97-111 (1994). [ Links ]
Received: November 13, 2000.
Accepted for publication: March 30, 2001.
Recommended by Subject Editor Ana Lea Cukierman.














