Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Latin American applied research
versión impresa ISSN 0327-0793
Lat. Am. appl. res. v.34 n.3 Bahía Blanca jul./sept. 2004
Effects of solution and soil chemistry on the distribution of oil residual in patagonian soil
S. M. Ríos2, N. S. Nudelman1 and O. Katusich2
1 Department of Organic Chemistry, Facultad de Ciencias Exactas y Naturales,
Universidad de Buenos Aires, 1428 Buenos Aires, Argentina
nudelman@qo.fcen.uba.ar
2 Department of Chemistry. Universidad Nacional de la Patagonia San Juan Bosco,
Comodoro Rivadavia, 9004 Chubut, Argentina
riossm@unpata.edu.ar
Abstract — The distribution coefficient of oil residuals between water and the soil under equilibrium conditions is, in the case of Patagonian soils, strongly dependent on the clay contents and humidity of the soil. Other variables such as, the soil salinity and the environmental exposure conditions, also affect the interactions between the phases. The oil residuals are generally accompanied by water spills, that are extracted together with the oil and which, frequently, have high salinity. These salts stay on soils during long times and finally they became part of the soil. The resulting oil aqueous concentrations and the soil-water distribution ratio (Kd) may be strongly influenced by these factors. Based on experimental data of several parameters determined in different regional field samplings, a semi-empiric model was developed that allows prediction of the Kd dependence with the exposure time, the salinity of the equilibrium aqueous concentration and the clay contents of the soil. The Monte Carlo simulation was used in this work. Distribution values of soil conductivity, clays content, and assumed fixed values of the age of the oil spill were used in the model. A set of calculated values of Kd was obtained and the results show a distribution that was probabilistically analyzed. The Kd values increase with increasing age of spill and soil salinity and decrease when the salinity of the initial aqueous concentration is greater than the soil salinity. The determined parameters are useful for modeling of the environmental impact on polluted soils and for the design of remediation techniques.
Keywords — Oil Residuals. Distribution Coefficient. Salinity Effect. Semi-Empiric Model. Monte Carlo Simulation.
I. INTRODUCTION
The behavior of the compounds in aqueous phase is of critical importance in environmental studies, because solute transport and transformations processes are know to occur predominantly in water (Lane and Loehr, 1992; Manan, 1996). Bioavailability of oil components in contaminated soils is an important regulating factor for biodegradation rates. At lower concentrations, the bioavailability was controlled by desorption/diffusion processes in water (De Jonge et al., 1997).
Sorption to natural solids is an underlying process affecting the transport, degradation, and biological activity of organic compounds in the environment (Pignatello and Baoshan, 1996). The overall sorption capacity is influenced by the nature of the soil organic matter, the mineral composition, the soil moisture content, and the presence of solvent. In dry soils, sorption of nonpolar organics in dry soils is dominated by adsorption onto mineral surfaces, particularly clays (Karimi-Loftfabad et al., 1996; Nudelman et al., 2000).
The simplest and most common method for mathematically expressing the distribution of an organic chemical between soil surfaces and water, is the sorption or distribution coefficient (Kd). Several researchers have found that if Kd is normalized on the basis of the soil's organic matter or organic carbon content, much of the variation observed among Kd's over different soils can be eliminated (Dragun, 1998). That there are differences in the sorption of organic compounds on different fractions of organic matter is known (Luthy et al., 1998). Others researchers have found that the extent of adsorption of nonionic organic chemicals onto soil particles surfaces is well-correlated with two empirical measurements of the organic chemical hydrophobicity: the water solubility and the octanol-water partition coefficient (Carmo, 2000; Xia, 2001). These available predictive expressions of Kd do not take into account the "salt" effect, the change of the adsorption coefficient due to a change in the salt content the water. The dependence of adsorption on salt content was aproximated with a derivative of the Setschenow equation (Dragun, 1998).
The oil residuals, in exploration and production areas, are generally accompanied by water spill which is extracted together with the oil and, frequently, has similar salinity to sea water. These salts stay on soils during long times and they became part of the soil. Removal of salts from oil and salt contaminated soils before undertaking bioremediation may reduce the time required for bioremediation (Rhykerd, 1995).
The purpose of the present study was to examine the salinity effect on the estimation of the distribution coefficient of oil residuals, due to spills of different ages. With the determined parameters, a semi-empiric model was developed to estimate Kd. The Montecarlo method was used to study the parametric sensitivity of Kd with respect to the contact time, clay content and salinity content.
II. METHODS
A. Sample Characteristics
Contaminated soil samples, product of oil spills in six different locations in the surroundings of Comodoro Rivadavia's city, were obtained. The oil spills are of different ages and environmental exposure conditions, and from different crude oil sources. In all cases, except for the samples 1 and 6, fertilization of the affected areas was carried out to improve the general conditions of the land. Table 1 summarizes some properties of the samples.
All samples were extracted from the surface, except in the case of sample 1 that was extracted from a depth of 20 cm to evaluate the most aged residuals. The contamination reached that depth, since on the same land more recent oil residuals were overturned. Therefore, the age of the old sample is approximate. The contaminated soils were air-dried, ground, and sieved with a 1.7 mm sieve. The total hydrocarbon determination was carried out by Soxhlet continuous extraction with methylene chloride for 24 h to 48 h, depending on the sample. After distillation of the methylene chloride, the oil concentration was analyzed using UV-Visible spectrophotometry (Nudelman et al., 2002).
Table 1. Description of oil contaminated soil samples

a extract 1:5 wt/wt, b μ S cm-1, 25oC.
The oil residues were analyzed by silica gel column chromatography, to separate group components (i.e. aromatic, aliphatic and polar compounds). The eluent solvents were hexane (30mL), benzene (30mL) and methanol/chloroform (1:1) (30mL). The portion remaining in the column contains the "asphaltenes" fraction (Nudelman et al., 2000).
B. Desorption Experiments
The desorption protocol was as follows. Aliquots of approximately 0.1 g of each soil sample were placed in 15 mL test tubes (at least by duplicate), 10 mL of pure water was added to each tube, making the soil solution ratio 1:100. Three experiments were carried out for each sample, two with calcium chloride and another without it. Calcium chloride was added to each tube (1 or 2mL) to achieve a matrix of 0.01 N CaCl2 to provide a initial ionic strength and minimize nonsettling particles (Lane and Loehr, 1992). The tubes containing the soil-solvent slurries were periodically shaken during seven days. The soil solution ratio and the total contact time were selected on the basis of previous studies. The tubes were sealed with plastic film and covered with aluminum foil to avoid light exposure and prevent photooxidation. After the required time had elapsed the supernatant solution was separated from the soil, and the solution centrifuged at 3000 rpm for 10 min. The supernatant was immediately withdrawn from each tube and the oil concentration was analyzed by using UV-Visible spectrophotometry. The oil concentration in the solid phase was determined by Soxhlet extraction and UV-Visible spectrophotometry. Desorption determinations were carried out at ambient temperature (22 ± 3oC). The electric conductivity of the aqueous phase was measured at the beginning (t=0) and at the end of each experiment (t=7 days).
C. Theoretical Framework
The aqueous solubility of the organic compound in the presence of dissolved salts can be expressed by the Setchenow equation. (Schwarzenbach et al., 1995):
log Sw salt = log Sw - Ks [salt] (1)
where Sw salt is the molar solubility in the presence of salts, Sw is the molar aqueous solubility, Ks (M -1) is the salting constant (which is a function of the hydrophobic surface area of the compound) and [salt] is the molar concentration of dissolved salts. This relationship has been used, for example, for the calculation of aqueous solubility of organic pollutants as chloroform, lindane and vinyl chloride in sea water (Schwarzenbach et al., 1995).
The dependence of adsorption on salt content was approximated with a derivative of the Setschenow equation,
log (Kd I2 / Kd I 1) = Ks (I2 - I1) (2)
where I1 and I2 are the aqueous salt concentrations, Ks is the Setschenow constant for a given salt (e.g. 0.16 L mol-1 for NaCl) and Kd is the adsorption coefficient (Dragun, 1998).
Eq. (2) is strictly valid only for a single solute, however, in this work the applicability of the equation is tested considering the oil residual as only one solute. The scope of the equation. will be also examined to evaluate the variation of Kd with the salinity. The aqueous concentration and the distribution coefficients, in this case are global values, and therefore they account for the interactions among the components in the mixture and for the overall interactions of each of them with the mineral matrix.
III. RESULTS AND DISCUSSION
The electric conductivity of the aqueous phase, C, is a good measure of the total ionic strength (the ionic exchange characteristic of the soil plus the added calcium chloride). A relationship of the type of Eq. (2) can be formulated between C and Kd,
ln (Kd / Kd0) = a (C - C0) (3)
where C is the electric conductivity of the aqueous phase (μS cm-1), C0 is the electric conductivity of the aqueous phase without Cl2Ca, Kd (L kg-1) is the distribution coefficient observed with C, Kd0 is the distribution coefficient observed with C0 and "a" (μS-1cm) is the slope of the straight line. Fig. 1 shows the plotted data, which empirical correllation is given by Eq. (3). A similar relationship between Eqs. (2) and (3) is observed.
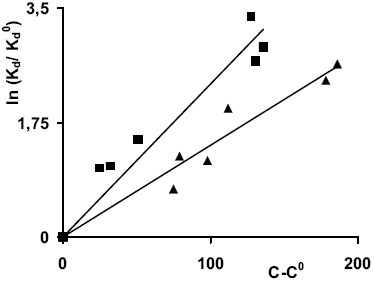
Fig. 1. Ln (Kd/Kd0) as a function of (C-C0), samples 1-3 (triangles) and samples 4-6 (squares). C and C0 are the electric conductivities of the aqueous phases, with and without Cl2Ca, respectively.
Samples 1-6 can be gathered in two groups: those with a ratio of Aliph/Pol (Aliphatic Groups/Polar Groups) near to 1 (samples 1-3) and those with ratios Aliph/Pol near to 0.5 (samples 4-6). The ratios are showed in Table 1. According to the model, an increase of sorption is observed when the ionic strength of the aqueous phase increases. The observed increase of Kd with the ionic strength agrees with the distributive behavior of the nonionic compounds depending on the changes in salinity (Young, 1992; Dragun, 1998). The slope of the straight line "a", is (1.33 ± 0.05) 10-2 μS-1cm, for residuals with ratios Aliph/Pol near to 1 (samples 1-3), and (1.92 ± 0.26) 10-2 μS-1cm for ratios Aliph/Pol near to 0.5 (samples 4-6). The regression values are r2 ≥ 0,923 in both cases. As it is observed for samples 1-3, an increase of the ratio Aliph/Pol is expected with age because these residuals have a higher hydrophobic behavior due to the loss of polar components with time. The increase of the slope "a" for samples 4-6 implies a high salinity effect on Kd, in agreement with the relative increase of polar compounds when the age decreases.
Prediction of the Kd Dependence
A semi-empiric model was developed that allows prediction of Kd as a function of the exposure time, the salinity of the equilibrium aqueous concentration and the soil clays content. The last variable was included because previous studies showed an important dependence of Kd with the clays soil content (Karimi et al., 1996), e.g. Kd (L kg-1) = 8.26 + 3.04 10-2 %clay, for dry soils, where %clay are the soil clay contents (%wt/wt). It is a correlation of experimental data obtained in organic phase (Nudelman et al., 2000).
The relationship (straight line) between the calculated (with the model) and the measured values (experimental data) of ln Kd, has a slope equal to 0.994 (r2 = 0.884). This slope indicates that ln Kd can be estimated with an error < 6%. Although the correlation coefficient is poor, it can be considered that a good adjustment has been achieved, taking into account the diversity in the environmental conditions, and the sources and history of the residuals.
Table 2. Simulations for assumed distributions of Ci, clays soil contents and Cs

X = mean, σ = standard deviation
To evaluate the sensitivity of the model to variations in the main factors involved in the prediction of Kd, the Monte Carlo simulation was used. Data of soil electric conductivity Cs, clays soil content (%wt/wt) and initial electric conductivity of the aqueous phase Ci were generated, according to the distributions assumed in the Table 2 (five different simulations). Each simulation described in this paper was the result of 100 repetitions. With the object to evaluate low, medium and high spill age, C, Kd0 and Kd have been calculated for oil residuals with spill age equal to 2, 10 and 20 years.

Fig. 2. Histograms showing results from Simulation 1.
(f% : percentage frequency)
Simulation 1. The results are shown in the Fig. 2. The aqueous salinity is less than the soil salinity. This situation could correspond to rain water that has increased its salinity during its superficial run-off. Mean values of electric conductivity have been assumed for soil salinity, according to regional data. The values of Kd (L kg-1) are ≤ 1000 for 2 year-old residuals (95%), while only 42% and 15% present these values for 10 and 20 years-old residuals, respectively. When the age of the spill increases, the maximum frequencies shift to higher values of Kd.

Fig. 3. Histograms showing results from Simulation 2.
(f% : percentage frequency)
Simulation 2. The results are shown in the Fig. 3. A higher electric conductivity for the soil has been assumed. When the soil salinity is greater than the aqueous salinity, Kd (L kg-1) increases and the maximum frequencies show at 1500 ≤ Kd ≤ 3000, for all the samples. Therefore, the age of spill is a secondary factor and the values of Kd would be mainly affected by the soil salinity.

Fig. 4. Histograms showing results from Simulation 3.
(f% : percentage frequency)
Simulation 3: The results are shown in the Fig. 4. In this case, the assumed mean value and standard deviation for Cs corresponds to regional sand-clay soils. It can be observed a decrease of Kd, due to the small clay soil content and a marked effect of age.

Fig. 5. Histograms showing results from Simulation 4.
(f% : percentage frequency)
Simulation 4. The results are shown in the Fig. 5. It has been assumed the Cs mean value and the standard deviation corresponding to regional clay soils. An increase in Kd, due to the high clay soil content can be observed. The distribution shows a bigger dispersion of the values as a function of the age.

Fig. 6. Histograms showing results from Simulation 5.
(f% : percentage frequency)
Simulation 5. The results are shown in the Fig. 6. For the initial aqueous phase salinity, a high mean value and standard deviation of Ci have been assumed. This situation could correspond to oil residuals that are accompanied by water spills, which are extracted together with the oil, which frequently, have salinity similar to sea water. When the initial aqueous salinity is greater than the soil salinity, a decrease in Kd is observed: 300 ≤ Kd ≤ 1200 for all the samples.
IV. CONCLUSIONS
The increase of Kd with increasing soil salinity (simulation 2), would imply a high degree of oil sorption under these conditions. This would agree with the observation that, when the soil salinity increases, the salinity of the equilibrium aqueous phase also increases and, therefore, the oil solubility decreases (Schwarzenbach et al., 1995). On the other hand, this effect is more important than the age. This same conclusion arises from the observation of a decrease in Kd when the initial aqueous phase salinity increases (simulation 5). Under these conditions the equilibrium aqueous phase salinity decrease (due to the adsorption of ions by soil) and this would imply an increase of the oil solubility in relation to the simulation 1.
An increase in Kd when increasing age of the residual, has been observed in all the simulations. However, the equilibrium aqueous phase salinity minimizes this effect, while the clays content makes the differences more evident (simulation 3 and 4). This is in agreement with our recent observations (Nudelman et al., 2000), that the increase of Kd with the clays content could, in principle, be attributed to an increase in the sorption area. Since a differential uptake is observed for the different fractions, this is interpreted as an indication of a strong specific interaction among polar components of the sorbate and the clays.
REFERENCES
1. Carmo, A.M., L.S. Hundal and M.L. Thompson, "Sorption of Hydrophobic Organic Compounds by Soil Materials: Application of Unit Equivalent Freundlich Coefficients", Environ. Sci. Technol., 34, 4363-4369 (2000). [ Links ]
2. De Jonge, H., J.I. Freijer, J.M. Verstraten and J. Westerveld, "Relation between Bioavailability and Fuel oil Hydrocarbon Composition in Contaminated Soils", Environ. Sci. Technol., 31, 771-775 (1997). [ Links ]
3. Dragun, J., The Soil Chemistry of Hazardous Materials, Amherst Scientific Publishers, 2da Edition, Massachusetts (1998). [ Links ]
4. Karimi-Lotfabad, S., M.A. Pickard and M.R. Gray, "Reactions of Polynuclear Aromatic Hydrocarbons on Soil", Environ. Sci. Technol., 30, 1145-1151(1996). [ Links ]
5. Lane, W.F. and R.C. Loehr, "Estimating the Equilibrium Aqueous Concentrations of Polynuclear Aromatic Hydrocarbons in Complex Mixtures", Environ. Sci. Technol., 26, 983-990 (1992). [ Links ]
6. Luthy, R.G., G.R. Aiken, M.L. Brusseau, S.D. Cunningham, P.M. Gschwend, J.J. Pignatello, M. Reinhard, S.J. Traina, W.J.Jr. Weber and J.C. Westall, "Sequestration of Hydrophobic Organic Contaminants by Geosorbents", Environ. Sci. Technol., 31, 3341-3347(1998). [ Links ]
7. Manan, S.E., Environmental Chemistry, Lewis Publishers, 6th Edition, Michigan, (1996). [ Links ]
8. Nudelman, N., S.M. Ríos and O. Katusich. "Interactions between Crude Oil and Patagonian Soil as a Function of the Clay-Water Contents", Environ. Technol., 21, 437-446 (2000). [ Links ]
9. Nudelman, N., S.M. Ríos and O. Katusich, "Organic Cosolvent Effect on the Estimation of the Equilibrium Aqueous Concentrations of Oil Residuals in Patagonian Soil", Environ. Technol., 23, 961-969 (2002). [ Links ]
10. Pignatello, J.J. and B. Xing, "Mechanisms of Slow Sorption of Organic Chemicals to Natural Particles", Environ. Sci. Technol., 30, 1-10 (1996). [ Links ]
11. Rhykerd, R.L., R.W. Weaver and K.J. McInnes, "Influence of Salinity on Bioremediation of Oil in Soil", Environmental Pollution, 90, 127-130 (1995). [ Links ]
12. Schwarzenbach, R.P., P.M. Gschwend and D.M. Imboden, Environmental Organic Chemistry, Wiley, NY (1995). [ Links ]
13. Xia, G., J.J. Pignatello, "Detailed Sorption Isotherms of Polar and Apolar Compounds in a High-Organic Soil", Environ. Sci. Technol., 35, 84-94 (2001). [ Links ]
14. Yong, R.N., A.M.O. Mohamed and B.P. Warkentin, Principles of Contaminant Transport in Soils, Elsevier, Amsterdam (1992). [ Links ]














