Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Latin American applied research
versión impresa ISSN 0327-0793
Lat. Am. appl. res. v.34 n.3 Bahía Blanca jul./sept. 2004
Optimal operating points in alkaline pulping
Vicente Costanza1*, Miguel A. Zanuttini2
1 Grupo Tecnología de la Madera, INTEC, UNL-CONICET, Güemes 3450, 3000 Santa Fe, Argentina.
Tel +54 342 4559174; Fax 4532965, e-mail:tsinoli@ceride.gov.ar
2 Instituto de Tecnología Celulósica, FIQ, UNL,
Santiago del Estero 2654, 3000 Santa Fe, Argentina. e-mail: mzanutti@fiqus.unl.edu.ar
Abstract — The problem of optimizing the alkaline impregnation of wood chips is posed and solved under usual restrictions. The cost to be optimized balances opposite criteria by taking economics and product quality into account, and is conditioned by the system dynamics. Evolution is modeled from typical transport phenomena equations. Optimization is attacked in the lines of variational calculus, although the final treatment involves numerical methods.
Cost function design is provided for: alkali consumption, thermal energy consumption, product quality, and total production; each one affected by a preference-weighting coefficient. A new parameter, the "Deacetylation Index", is introduced as an observable quantity for tracking the end of the digestion stage in pulping processes. This index turns out to be significant even at low temperatures. Cost terms depend essentially on three design variables: (i) alkaline bulk concentration, (ii) digester temperature, and (iii) total duration of the process. An algorithm to ascertain the optimal values of these variables is devised. Numerical results provide insight in deciding changes on design variables in case they are allowed within a certain extent to be manipulated.
Keywords — Optimization. Transport Processes. Alkaline Pulping.
* Autor to whom the correspondence should be addressed.
I. INTRODUCTION
A proper penetration and/or diffusion of alkali into wood chips is essential for all alkaline pulping processes, not only in high yield chemimechanical but for chemical or semichemical pulping as well.
The arriving of the alkali produces swelling and softening of the wood. Both effects are necessary in the ulterior defibration stage of a chemimechanical pulping (Heitner and Atack, 1983). On the other hand, the quality of the impregnation stage previous digestion affects the homogeneity of the chemical treatment, which in turn has incidence on the rejects content of the pulp and final paper quality.
Wood impregnation involves not only diffusion but also, in the case of alkaline liquors, chemical reaction. Especially under moderate conditions, such as those found in chemimechanical alkaline processes, any modeling approach must take into account reaction mechanisms since reagent concentration and load are relatively low and the alkali may be totally consumed by these reactions.
The alkali deacetylates the wood and activates carboxylic groups of the hemicellulose. According to previous work (Zanuttini et al., 1999), the quality of chemimechanical pulps after alkaline treatment is more sensitive to deacetylation than to carboxylic group content.
Traditionally, in alkaline chemical pulpings impregnation is conducted at temperatures above 100 C. High temperature results in an overall diffusion-controlled process with flat reaction profiles in agreement with classical unreacted-core model results. For short reaction times, this leads to mostly unreacted chips. Ideally, reaction should occur as soon as the alkali reaches acetyl groups. It is therefore desired for the penetration of the alkali into the wood to be reaction-driven, which only happens at high reaction rates. Perhaps one of the reasons why some researchers (Zanuttini et al., 1999, Jimenez et al., 1989a,b, Jimenez et al., 1990) adopted the travelling front model was their familiarity with wood preserving pressure treatments, where diffusion is practically ignored (Costanza and Miyara, 1988, 2000).
At low temperatures alkali diffusion becomes substantial, the process is no longer diffusion-controlled, and there is a reaction gradient inside the chip, so reaction occurs in all parts of the wood, leaving no unreacted core.
Previous experimental work on the pure diffusion mechanism deserved criticism. McKibbins (1960), and Lönnberg and Robertsen (1992) did not distinguish between diffusion alone and diffusion combined with chemical reaction, so they obtained not really pure but combined coefficients. (See Costanza and Costanza, 2002 for an uncoupled treatment). Talton and Cornell (1987) measured diffusion in the reverse direction, i.e. from saturated wood to dilute solution, without commenting on the eventual distortion due to hysteresis effects. A simplified modeling approach studied the uncoupled system which considered a mass balance for the alkali alone (Kazi et al., 1997). The difference observed between predicted and experimentally obtained alkali concentration profiles suggested that the diffusion coefficient is not a constant but a certain function of the alkali concentration.
In our approach, the variation of the diffusion coefficient was first confirmed by experiments and then, taking into account the effect of concentration changes, coupled mass balance equations for both alkali and acetyl species were advanced (Costanza et al., 2001). Comparisons between predicted values and experimental results were performed in terms of two parameters: "Penetration" and "Deacetylation Index".
Based in the coupled model, a numerical procedure for the static optimization of the alkaline batch digestion of wood chips is presented here. An optimal set ("control" in a generalized sense) is defined as a 3-components vector containing values for the design variables which minimize the performance measure (Kelso et al.,1963, Siau, 1984, Intriligator, 1971). In this work, cost is the performance measure to be minimized, involving four (eventually antagonistic) summands: alkali consumption, thermal energy consumption, product quality, and gross production; each one affected by a preference-weighting coefficient.
The cost to optimize is restricted by the system dynamics, in the sense that each of the individual contributions depends on final values for the batch process. To predict these final values, system dynamics are modeled through an adaptation of classic transport phenomena equations to the special features of this process, here discovered and/or discussed. The parameter Deacetylation Index was devised as an observable measure useful for tracking the end of the impregnation stage, and characterizes product quality. Cost terms depend on: (i) alkaline bath concentration, (ii) digester temperature, and (iii) total process time.
II. MODEL DESCRIPTION
A. Dynamical Model
A spatially one-dimensional (along the x-direction, see Fig. 1) mass-balance model was used for the two main components: NaOH and Acetyl groups.
Typical values for chip thickness are 3-6 mm, and the length-to-thickness ratio is about 5:1 or higher. In alkaline pulping the diffusivities in the three primary directions are very similar (Hartler, 1962, Rydholm, 1965, Akhtaruzzaman and Virkola, 1979); therefore, chip thickness will be the critical dimension when treating the diffusion mechanism.
The isothermal mass conservation equations governing alkali penetration are (Bird et al.,1960, Grindrod, 1996)
 | (1) |
 | (2) |
where C is the alkali concentration, X is the acetyl content in wood, D is the diffusion coefficient, and r is the reaction rate. In what follows, the functional dependence of the reaction rate on C and X will be assumed to be of the form
r(C,X) = k Cλ Xξ (3)
validated in previous work (Zanuttini and Marzocchi, 1997).

Figure 1. Adopted geometry.
Initial and boundary conditions are:
 (no mass flux in the center of the chip at all times)
(no mass flux in the center of the chip at all times)
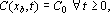 (no border diffusion restriction).
(no border diffusion restriction).
C0: initial alkali concentration in the bulk,
xb: half-thickness of the chip,
Under similar conditions this was assumed by several authors (Lönnberg and Robertsen, 1992, McKibbins, 1960). Furthermore, experimental results justify this assumption (Costanza and Costanza, 2002, Fig. 5).
 ("virgin wood")
("virgin wood")

X0 initial acetyl content in the wood chip
From Eqs. (1) and (2), alkali concentration inside the chip changes due to diffusion but also because of chemical reaction with acetyl groups. On the other hand, the concentration of acetyl groups (which do not diffuse, since they are part of the solid structure of the wood chip) decreases by effect of chemical reaction with the alkali only.
Kazi et al. (1997) treated Eq. (1) alone, assuming that the chemical reaction is irrelevant and proposing a Bessel-function solution for the pure-diffusion situation. Recent experiments show that acetyl groups consumption actually has an important effect over the alkali concentration evolution inside the wood chip, and then a more complete model must be considered.
The following hypotheses are used in the theoretical and numerical treatment of the equations:
(i) "Expression (3)" for the reaction rate is adopted with (Zanuttini and Marzocchi, 1997) λ = 1.35; ξ = 2. This reaction rate is nonlinear, which seriously hinders the analytical treatment of the differential equations. It was shown (Zanuttini et al., 1999), that this reaction accounts for most of the alkali consumption, therefore the reaction is here considered as mole-to-mole.
(ii) Only isothermal processes will be considered, and the whole chip is assumed to have the same temperature as the bulk of the solution at all times.
(iii) The reaction's kinetic constant follows the behavior predicted by Arrhenius with respect to temperature, and its constants were experimentally found by Zanuttini and Marzocchi (1997).
 [%(ξ -1) /((g/L)λ min)] (5)
[%(ξ -1) /((g/L)λ min)] (5)
(iv) The diffusion coefficient D is a practically linear function of temperature (Zanuttini and Marzocchi, 1997)
 | [cm2 / min] (6) |
where D(T) is the notation for the diffusion coefficient at temperature T.
(v) Experimental evidence and physical constraints suggest a sigmoid functionality for D with alkali concentration (see Fig. 4). Under such hypothesis, an almost constant diffusion coefficient would be valid in the range of high alkali concentrations (usually near the boundary), another one in the low concentration range (near the center of the chip), and there will usually exist a significant gradient inside the chip.
In the regions where an almost constant D can be assumed, Eq. (1) can be rewritten as
 | (7) |
A qualitative study of Eq. (7) starts by observing that Sign = Sign
= Sign . Since
. Since  and r(C, X) are always positive, then
and r(C, X) are always positive, then  must be always greater than 0, as in the pure diffusion case.
must be always greater than 0, as in the pure diffusion case.
For certain bulk concentration conditions, experimental alkali profiles show that  for a time-varying dependent position xo(t). If D was a constant then Eq. (7) solution should show that
for a time-varying dependent position xo(t). If D was a constant then Eq. (7) solution should show that  at (t, xo). The positiveness of
at (t, xo). The positiveness of  and r(C,X) makes this impossible. This dilemma can be solved by returning to the general form of Eq. (1), where the diffusion coefficient is a function of the alkali concentration. Rewriting Eq. (1) as
and r(C,X) makes this impossible. This dilemma can be solved by returning to the general form of Eq. (1), where the diffusion coefficient is a function of the alkali concentration. Rewriting Eq. (1) as
 | (8) |
it is easy to see that the fact that  for some
for some
(t, xo) would imply that  , which poses a restriction on the qualitative dependence of the parameter D.
, which poses a restriction on the qualitative dependence of the parameter D.
Eqs. (8) and (2) conform a nonlinear coupled system of PDE's whose analytical solution is unknown (Bretsznajder, 1971, Britton, 1986). Simulations were then performed using standard numerical methods for PDE's.
It should be noticed that the model described here would not respond accurately for much thicker wood samples due to the inhomogeneity of wood, air entrapped in the vessels, etc. (Kelso et al., 1963). Also, in an industrial context, wood chips might come from different sources and with varying moisture contents, and they might be stored in variable conditions during several days before digestion. Here it was assumed that chips were kept in an aqueous bath until water penetrated into the entire material, leaving no space occupied by gases.
B. Optimization Set-up
The cost function J proposed in this work is the addition of four terms Ci, each one multiplied by a weight coefficient wi (the set of wi's normalized to unity)
J = wp Cp + wa Ca + we Ce + wc Cc (9)
The Ci's are costs in monetary units associated to each economic contribution, namely: Cp is the production cost, Ca is the alkali reactant cost, Ce is the thermal energy cost, and Cc is the quality cost. This Ci's have the following expressions in terms of the design variables and other parameters:
(i) (10)
(10)
where tf is the residence time in the digester. Clearly, productivity is equivalent to the duration of the batch process: the smaller this duration is, the largest is the total production output of the plant per unit time since the size of the batch reactor and its charge are kept constant. Here and in what follows the α i's coefficients are conversion-to-monetary-unit factors: α p converts time units to money, and varies according with the type of product the plant is producing.
| (ii) |  | (11) |
Where the α's account for twice the half-thickness xb, C(x,t) is the alkali concentration inside the chip as a function of time and position coming from simulations of the dynamic model, analogously X(x,t) is the acetyl groups content inside the chip, and α 1a and α 2a are alkali mass units converters. Eq. (11) indicates that the total alkali consumed during the process is the sum of two terms: the first one is the total alkali remaining in the chip at final time; the second one is the alkali that has already reacted, measured in terms of the same number of neutralized acetyl groups. The minus sign appears because X(x,t) is the concentration of acetyl groups at time t, which is transformed into reacted acetyl groups and finally into alkali mass units via α 2a (see below).
(iii)  (12)
(12)
where Tf is the bulk temperature in the digester, T0 is the temperature at which the mixture is introduced in the digester, Te is the ambient temperature, and α 1e and α 2e are energy units conversion factors. The energy cost is then expressed also as the sum of two terms, the first one models the effort needed to reach the temperature of operation after the mixture is introduced in the digester (t = 0), and the second one accounts for the heat needed to maintain this temperature of operation afterwards (until t = tf).
The last cost factor is related to product quality, which is considered in this paper to be equivalent to the amount of acetyl groups that the process was not able to neutralize, i.e.
(iv)  (13)
(13)
where αc converts acetyl groups mass units to monetary units. (αc includes the factor 2 due to xb).
Detailed expressions for the α coefficients are
 | , |  | (14, 15) |
 | , |  | (16, 17) |
 | , |  | (18, 19) |
where h is the heat-transfer film coefficient, A is the interfacial area,  is the average specific heat of the solution and chips mixture, X0 is the initial acetyl groups content inside the chip before digestion, πp is the cost per unit mass of product, πa is the cost per alkali unit mass, πe is the cost of the calorie, πc is the extra profit per point obtained in deacetilation, M is the total mass of chips, m is the average mass of one chip, P is the total mass of the mixture (chips and solution), and s is a constant relating alkali and acetyl groups units (0.139 in this case) (Zanuttini and Marzocchi, 1997).
is the average specific heat of the solution and chips mixture, X0 is the initial acetyl groups content inside the chip before digestion, πp is the cost per unit mass of product, πa is the cost per alkali unit mass, πe is the cost of the calorie, πc is the extra profit per point obtained in deacetilation, M is the total mass of chips, m is the average mass of one chip, P is the total mass of the mixture (chips and solution), and s is a constant relating alkali and acetyl groups units (0.139 in this case) (Zanuttini and Marzocchi, 1997).
In order to obtain demonstrative results, "Expression (9)" was evaluated for chosen sets of α i's and wi's (see Fig. 12). Cost function J has relative minima, and an absolute minimum can always be obtained. The absolute minimum is a 3-component vector (t, T, C0) depending on the α i's and the wi's considered, i.e., it is an optimal operating point.
III. EXPERIMENTAL
A. Wood
Wood studied was a specially-produced variety of Carolina poplar (populus deltoides carolinensis). Samples were provided by a local paper-manufacturing firm in the form of fresh logs that were cut into 2.5 cm-thick slices and preserved in a freezer.
B. Diffusion Coefficient Measurements
McKibbins (1960) and Lönnberg and Robertsen (1992) introduce fresh chips into alkali solution in order to assess diffusion effects, and do not take into account the fact that at the same time that the alkali is diffusing into the wood, it is also reacting with acetyl groups. Therefore, (alkali) concentration variations are due not only to diffusion but also to chemical reaction, and the measurements obtained cannot distinguish between these mechanisms. Talton and Cornell (1987) neglect eventual hysteresis effects.
In Costanza and Costanza (2002), in order to perform additional mesurements needed here, an accurate experimental apparatus was adopted. The cell consists of two chambers connected by a square opening in which a wood chip fits. The only possible diffusion path is between chambers and through the piece of wood. Both chambers are provided with stirrers so as to eliminate concentration gradients within them. One of the chambers is loaded with a concentrated alkali solution, while the other is filled with a dilute solution. The dilute side chamber is equipped with a conductivity measurement cell and a temperature compensation probe. Both chambers are also provided with N2 inlets in order to generate an inert atmosphere and avoid alkali reaction with CO2 from the surroundings.
Experiments were conducted under conditions ensuring that: (i) there exists only a molecular type of diffusion through the wood chip, (ii) the chemical reaction is already completed (deacetylated wood), and (iii) convective flow can be neglected. Then for each value of time t, the Fick's law of diffusion applies:
 (20)
(20)
where N is the total alkali molar flow velocity towards the inside of the wood chip, JD is the alkali molar diffusive flow velocity, and ∇ C is the alkali concentration gradient in the wood chip.
Since JD is by definition, 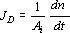 , n = C V, where Ai is the interface area of the wood chip in the experimental diffusion cell, V is the diffusion cell volume, and n is the number of alkali moles; and assuming that V is constant in time, then
, n = C V, where Ai is the interface area of the wood chip in the experimental diffusion cell, V is the diffusion cell volume, and n is the number of alkali moles; and assuming that V is constant in time, then
 | (21) |
Since diffusion takes place mostly in only one direction, the gradient may be replaced by the x-derivative of concentration. The assumption that the chip is very "thin" allows to approximate the derivative by the incremental ratio ∂C/∂x. The time derivatives of ∂C and of CB (the measured alkali concentration of solution in the dilute side compartment of the diffusion cell) are the same since the bulk concentration CA is kept constant. This reasoning leads to a linear ODE for CB, whose solution yields the concise expression
 | (22) |
where xb is half the thickness of the wood chip,  is the slope of the experimental points at t = 0 (discarding initial noisy data), and
is the slope of the experimental points at t = 0 (discarding initial noisy data), and  is the initial alkali concentration of solution in the concentrated side compartment of the diffusion cell.
is the initial alkali concentration of solution in the concentrated side compartment of the diffusion cell.
Wood slices were cut and polished into chips of nearly 30 x 30 x 1.5 mm. Chips were kept in water for 48 hours in order to minimize distortions due to air entrapped in the vessels. Afterwards they were fastened to polypropylene supports with an ordinary adhesive. Sample stands were in turn fastened to the cell partition wall assuring both compartments were watertight. Solutions were loaded into each compartment and conductivity vs. time data recorded. The process was allowed to continue during enough time to assure that the chemical reaction was complete. This was checked by tracking the slope of the conductivity vs. time charts. A constant slope observed in successive experiments indicates that the chemical reaction is complete, and thus that experimental data are directly related to the pure diffusion mechanism. In this condition, conductivity data are converted into concentration data, and Eq. (22) is solved for the particular experimental conditions.
The resulting diffusion coefficient for 6.0g NaOH/L is D = 4.4 10-4 cm2/min, which is comparable to values for similar materials (see Bird et al., 1960). Other measurements are plotted on Fig. 4.
C. Determination of the alkali concentration and penetration profiles
Wood cubes were cut from the slices by means of a microtome, to assure parallel planes in the relevant faces. Then, the cubes were placed in a water bath and vacuum up to 30 mmHg. Under these conditions cubes sank in just a few minutes releasing air bubbles for some hours. The process was carried on during enough time for all the air to be evacuated. Before alkaline treatment, the other four faces were sealed so as to prevent alkaline attack in the radial and axial directions, taking special care to prevent the wood from drying out. In order to ensure that the material's treatment was isothermal, the cubes were preheated in a microwave oven with maximum temperature control. The same control was performed on other similar cubes obtained from the same log section, which were drilled to allow the insertion of a temperature probe; these latter cubes were used as standards. Each cube was wrapped in polyethylene film to prevent moisture losses during preheating. Once unwrapped, the preheated cubes were quickly immersed into the thermostat containing the liquor. The alkaline treatments here discussed were conducted under the following conditions: T = 363 K (90oC); C = 5 g/L; t = 10, 40, 80, 120, and 180 minutes.
Final treatment time was longer than usual for industrial chemimechanical pulping, to allow for a better tracking of the phenomenon's patterns. Once each treatment time was reached, the cubes were immersed into liquid nitrogen for at least two hours to quell the reaction. The cubes were then stored in a freezer at -20 oC. For chemical analysis 100-µm-thick slices were cut from the frozen cubes, except in those cases in which the material had been excessively softened, where thickness was increased to 200 µm to preserve slices' integrity. The slices were immediately weighed, then submerged in 20-mL water and quantitatively neutralized to determine their alkaline load with 0.005 M HCl; this process carried on during enough time to permit alkali diffusion into the neutralizing environment, which was indicated by the phenolphthalein coloration. The dry weighing of the slices allowed their liquid content to be determined and expressed as g liquid/g treated wood, and the alkali concentration as g NaOH/L liquid. The slices' acetyl content was then determined by means of a gas-liquid chromatography technique. The alkali content of the slices defined the limit depth of the penetration that had taken place. Alkali concentration experimental results are shown in Fig. 2. Penetration experimental points are depicted in Fig. 7.

Figure 2. Experimental alkali concentration points
The penetration distance considered in each case corresponded to the central plane of each slice.
D. Determination of the acetyl groups content
The method adopted for assessing acetyl group content comprises two stages: (i) deacetylation in oxalic acid, and (ii) determination of the acetic acid produced during the first stage through gas-liquid chromatography. The last step uses propionic acid as an internal standard, as proposed by Solár et al. (1987).
The samples analyzed here (micrometric slices) have a very low weight, in the order of 20-50 mg, instead of the 300 mg in the original technique proposed by Solár et al. (1987). Each slice is cut into small pieces and placed in a 2 mL cruet; a volume of 1 mL of digestion liquor consisting of oxalic acid in saturation and propionic acid (1 g/L) is then added. Digestion in the closed cruet was allowed to proceed for 50 minutes at a temperature of 152 oC; under these conditions, total hydrolysis of the acetyl groups was achieved. After cooling, the digestion liquor in each cruet was poured into a vial for storage and later analysis.
The acetic acid content in the liquor was then determined in relation to the propionic acid concentration. The acetyl content in each sample was then calculated from the acetic acid concentration and expressed as mass of acetyl groups/mass of treated wood. Acetyl content experimental results are shown in Fig. 3.

Figure 3. Experimental Acetyl content points
IV. NUMERICAL RESULTS
A. Simulation of the Dynamical Model
Concerning the prediction of complete alkali concentration and acetyl content profiles, the importance of the diffusion effects is affected by variations in bulk concentration. Based on Stone's work (Stone, 1957), and the qualitative study performed on Eq. (8), a sigmoid functionality of the diffusion coefficient D with alkali concentration interpolates the experimental results as shown in Fig. 4. The sigmoid is chosen because it reflects the existence of thresholds for the diffusion coefficient at high and low concentrations.

Figure 4. Diffusion coefficient as a function of alkali concentration
Since the algebraic system of Eqs. (8) and (2) is not elliptical and is not expected to have singularities, the so-called numerical "Line Method" was used to solve the PDE's. This method consists of discretizing one variable (position in this case) to obtain a system of coupled ODE's in the time variable. The resulting nonlinear system is in turn solved iteratively until results converge. The numerical convergence is assured adopting a relative error lower than 10-5 for both spatial and temporal domains. Figs. 5 and 6 show the resulting alkali and acetyl profiles for parameters, initial conditions and boundary conditions actually used in the pulping industry: T = 363 K (90 oC); Xo = 3.22 % in weight on a dry wood (constant weight) basis; Co = 5 g/L constant bulk NaOH concentration; k = 0,427 %(ξ - 1) /((g/l)λ min) (from Eq. (5)).
It is clearly seen in Figs. 5 and 6 that simulated alkali concentration and acetyl content profiles qualitatively agree with the experimental points of Figs. 2 and 3, thus validating the model to a reasonable extent.
Acetyl content profiles (Fig. 6) present a "reaction zone" whose width is defined by the reaction rate (and by temperature, since the reaction constant follows Arrhenius' law). This zone can be thought of as the one comprised between 0.9 and 0.1 of the initial acetyl content of the wood sample. Thus, the reaction penetrates into the chip at a higher rate at the beginning of the impregnation, then tends to level off as it reaches the chip core.

Figure 5. Simulation Results: Alkali concentration profiles

Figure 6. Simulation Results: Acetyl content profiles
The reagent's "penetration" as a function of time t has been defined as the point x(t) for which the alkali concentration in the wood chip reaches 50% (or a suitable choice) of the initial alkali concentration in the bulk. With this definition, it is possible to associate penetration with the degree of deacetylation attained by the wood sample. The time required for the alkali to reach the chip core, which corresponds to a state of "total" deacetylation of the chip, can also be readily obtained. Knowledge of this time is of a paramount economic importance in the pulping industry because it defines the digestion time, or the residence time of the load in the reactor. In Fig. 7 "penetration" is taken to be the point at which alkali concentration has reached 50% of the original bulk value. The points were obtained as described in the Experimental Section.

Figure 7. Simulated alkali penetration p[mm] time curve vs. Experimental points
Fig. 7 indicates that the model predicts not only the qualitative form of the curve, but also penetration quantitative values. The fact that experimental points are slightly under the theoretically predicted curve can be attributed to secondary reactions not considered in the mass balance equations. There might also be some systematic errors in the experimental procedure due to, for instance, initial (after soaking) chip moisture content slightly under total saturation point.

Figure 8a. Simulation Results: Deacetylation Index and alkali penetration re-scaled (p/xb). T = 363 K (90oC)
The results predicted by the model can also be expressed in terms of the Deacetylation Index, which is defined as the reached fraction of the total chip deacetylation, i.e., the total acetyl groups consumed by the reaction divided by their initial contents in wood:
 | (23) |
This representation has the practical advantage of indicating at every instant the proportion of consumed and remaining acetyl groups in the chip. As announced, deacetylation describes the quality of the product better than carboxylic groups content. Deacetylation Index as a function of time is depicted in Fig. 8a,b; together with re-scaled (p/xb) alkali penetration curves for different percentages used in the definition.

Figure 8b. Simulation Results: Deacetylation Index and alkali penetration re-scaled (p/xb). T = 298 K (25oC)
Comparison between Deacetylation Index and alkali penetration curves shows that both parameters are qualitatively similar. From the quantitative point of view, the Deacetylation Index is similar to a re-scaled small percentage of the alkali penetration curve. Even when little alkali is remaining at a given point (i.e., penetration is low), a significant amount may have arrived there via diffusion and deacetylated correspondingly much of the wood (high consumption of alkali due to reaction). Numerical results emphasize the fact that deacetylation is significant even at low penetrations and temperatures. Consequently, penetration measured in the conventional way does not efficiently depict the relevant aspect of the system's evolution. Clearly, the quality of the resulting pulp will be better predicted from the deacetylation status than from the alkali content remaining after reaction. Since penetration has been used extensively in industrial practice instead of deacetylation measures, the previous comments may partially explain the fact that diffusion effects have not been fully exploited up to the moment.
B. Optimization Calculations

Figure 9. Cost J as a function of time and temperature.



Figure 10. Level curves for cost function J.
The optimization problem, in the context of previous sections, is: to find the impregnation time t*, alkaline bath temperature T*, and alkali bulk concentration C0*, that minimize the objective cost function J for the batch period, under a fixed set of α i's and wi's coming from reliable industrial-financial data. Computations of "Expression (9)" for an alkaline chemimechanical pulping show several relative critical points, and almost always an absolute minimum in the same set (see Figs. 9 and 10).
The absolute minimum vector (t, T, C0)* depends on the form of the individual cost functions Ci's adopted, and their corresponding wi's.

Figure 11 a. Optimal Cost function J points in the temperature-time plane
Additional illustrative information may be obtained by varying the preference of just a cost "j" while keeping the other three equal,
 | ,  i: 1 to 4 i: 1 to 4 | , (24) |
 | , | (25) |
and plotting the points of absolute minimums of J connected by dot lines. Each of the Figs. 11 a through c are 2-dimensional projections showing the cost variations for the different j's.
An analysis of Figs. 11 a-c indicates that:
If the benefit for improving the quality of the final product takes relatively more importance in the global evaluation, then, in order to maintain optimal working conditions it will be convenient: (i) to increase the temperature in the digester, (ii) to reduce the total
residence time per batch, and (iii) to slightly reduce alkali concentration in the bulk.

Figure 11 b. Optimal Cost function J points in the alkali concentration-time plane

Figure 11 c. Optimal Cost function J points in the alkali concentration-temperature plane
When the cost/benefit of gross production increases in relation to the other components, then it will be appropriate: (i) to linearly reduce total residence time in the digester, (ii) to linearly increase the temperature, and (iii) to keep alkali concentration in the bulk nearly constant.
Provided the cost of the energy consumed in the process increases its incidence, then it is recommended: (i) to reduce the temperature in the digester, and (ii) to increase both the total residence time and the alkali concentration of the bulk.
Finally, in case the cost of the alkali increases, then (i) increase the temperature in the digester, (ii) linearly reduce the alkali concentration of the bulk, and (iii) keep the total residence time nearly constant.
Fig. 12 shows the optimal J as a function of each of the wi's of "Expression (9)". It is clearly seen that optimal values for cost function J strongly depend on the "quality" relative weight, while it shows less sensibility to changes in the other relative weights.

Figure 12. Cost function J for relative weights of energy, production, quality, and alkali concentration.
It should be noticed that different results (Figs. 9 and 10) would be obtained for another set of α i's and wi's, and that the validation of the whole optimization model is subjected to the availability of reliable industrial-financial data for these coefficients.
V. CONCLUSION
The results in this paper allow to predict and optimize the evolution of main variables during isothermal impregnation of chips of poplar wood. The optimization procedure proposed in this work is simple and concise. The problem, in the context described in the text, reduces to finding the impregnation time, alkaline bath temperature, and alkali bulk concentration, that minimize the objective cost function per batch period. Results obtained through numerical simulations take a few minutes in a regular desktop PC and provide complete information concerning advantageous regions of operation and optimization strategies.
Advances in modeling and simulation with respect to previous literature are also noticeable. The "deacetylation index" results in a more precise concept than conventional "penetration" to measure the extent of evolution of the process. Up to now the point of total or optimal impregnation extent was empirical knowledge, and tuning efforts had to be spent whenever changes in operating conditions were decided: temperature, alkali concentration, wood species, initial moisture, chip dimensions, etc. Equations with a position- and time-dependent diffusion coefficient can be eventually solved through the proposed numerical scheme, in case alkali-induced changes in wood structure (swelling) affect the diffusion coefficient in a measurable way. This tool is a first step towards industry-oriented utility programs for estimating total "cooking" times in the digester.
The set-up designed to treat static optimization provides also a comprehensive framework for the consideration of the optimal control of the alkaline digestion stage. The validation of the whole procedure depends on reliable industrial-financial data for the coefficients involved, for which an open attitude will be required of the pulping industry. Also, extensions to continuos processes, as well as the consideration of non-isothermal and concentration-dependent reaction rates are possible, and will in fact be the indicated matter for future study.
NOMENCLATURE
α p = Conversion coefficient between time units and monetary units.
α 1a, α 2a = Conversion coefficient between alkali mass units and monetary units.
α 1e, α 2e = Conversion coefficient between energy units and monetary units.
α c = Conversion coefficient between acetyl groups mass units and monetary units.
A = Interfacial area of the digester, m2.
Ai = Interfacial area of the wood chip in the experimental diffusion cell, cm2.
C(x,t) = Alkali concentration inside the chip as a function of time and position given by the dynamic model, g/L.
CA = Alkali concentration of solution in the concentrated side compartment of diffusion cell, g/L.
 = Initial alkali concentration in concentrated side compartment of the diffusion cell, g/L.
= Initial alkali concentration in concentrated side compartment of the diffusion cell, g/L.
CB = Measured alkali concentration of solution in the dilute side compartment of the cell, g/L.
 = slope of the experimental points at t = 0, g/(L min).
= slope of the experimental points at t = 0, g/(L min).
C0 = Bulk initial alkali concentration, g/L.
 = Average specific heat of the solution and chips mixture, cal/(Kg oC).
= Average specific heat of the solution and chips mixture, cal/(Kg oC).
Cp = Production cost, $.
Ca = Alkali cost, $.
Ce = energy cost, $.
Cc = Quality cost, $.
∇ C = Alkali concentration gradient in the wood chip, g/(L cm).
D = Radial alkali diffusion coefficient in wood, cm2/min.
J = Cost function, $.
JD = Alkali molar diffusive flow velocity, Mole/(cm2 min).
k = Reaction kinetic constant, %(ξ - 1) /((g/l)λ min).
h = Heat-transfer film coefficient, cal/(m2 min.).
M = Total mass of chips, Kg.
m = Average mass of one chip, Kg.
n = number of alkali moles
N = Total alkali molar flow velocity, Mole/(cm2 min).
P = Total mass of the mixture (chips and solution), Kg.
π p = Cost per product mass unit, $/Kg.
π a = Cost per alkali mass unit, $/kg of NaOH.
π e = Cost of the calorie, $/cal.
π c = Extra profit per deacetilation point obtained, $/deacetilation units.
r = Reaction rate, %/min.
s = Constant relating alkali and acetyl groups units, 0.139 in this case.
t = Time, min.
tf = Residence time in the digester, min.
T = Temperature, oK.
Te = Temperature of the environment, oC.
T0 = Temperature at which the mixture is introduced in the digester, oC.
Tf = Bulk temperature in the digester, oC.
V = Diffusion cell volume, cm3.
x = Position in radial direction of the wood chip, cm.
xb = Half thickness of the wood chip, cm.
X = Acetyl content, % in weight on a dry wood basis.
X(x,t) = Acetyl groups content inside the chip as a function of time and position, % in weight on a dry wood basis.
X0 = Initial acetyl content in the wood chip, % in weight on dry wood basis.
REFERENCES
1. Akhtaruzzaman, A.F., N.E. Virkola, "Influence of chip dimensions in Kraft pulping". Paperi ja Puu, 62(10), 607-612 (1979). [ Links ]
2. Bird, R.B., W.E. Stewart, E.N. Lightfoot, Transport Phenomena. Wiley, New York (1960). [ Links ]
3. Bretsznajder, S., Prediction of Transport and other physical properties of fluids. Pergamon Press, London (1971). [ Links ]
4. Britton, N.F., Reaction-Diffusion Equations and Their Applications to Biology, Academic Press, London (1986). [ Links ]
5. Costanza, V., P. Costanza, "Estimating Pure Diffusion Contributions in Alkaline Pulping Processes", Latin Amer. Applied Res., 32(2), 151-159 (2002). [ Links ]
6. Costanza, V., A.J. Miyara, "Modelización Parcial del Proceso de Impregnación de Maderas". Actas VI Congreso Forestal Argentino II, 591-603 (In Spanish) (1988). [ Links ]
7. Costanza, V., A.J. Miyara, "Dynamical Aspects of Hardwood Impregnation", Holzforschung, 54 (2), 183-187 (2000). [ Links ]
8. Costanza, V., F. Rossi, P. Costanza, M.A. Zanuttini, "Diffusion and Reaction in Isothermal Pulping Digesters", Ind Eng Chem Res., 40, 3965-72 (2001). [ Links ]
9. Grindrod, P., The Theory and Applications of Reaction-Diffusion Equations. Clarendon P., Oxford (1996). [ Links ]
10. Hartler, N., "Penetration and diffusion in Sulfate Cooking", Paperi ja Puu, 44(7), 365-370 (1962). [ Links ]
11. Heitner, C., D. Atack, "Ultra-high-yield pulping of Aspen, effects of ion content", Pulp and Paper Mag. Can, 84, 11-17 (1983). [ Links ]
12. Intriligator, M.D., Mathematical Optimization and Economic Theory. Prentice-Hall, New Jersey (1971). [ Links ]
13. Jimenez, G., R.R. Gustafson, W.T. McKean, "Modeling Incomplete Penetration of Kraft Pulping Liquor". J. Pulp and Paper Sci., 15 (3), 110-115 (1989a). [ Links ]
14. Jimenez, G., R.R. Gustafson, W.T. McKean, D. S. Chian, "The role of penetration and diffusion in nonuniform pulping of softwood chips". Tappi J., 72(8), 163-167 (1989b). [ Links ]
15. Jimenez, G., R.R. Gustafson, W.T. McKean, "Using a Kraft pulping model to improve pulp uniformity". Tappi J. 73(3), 173-176 (1990). [ Links ]
16. Kazi, K.M.F., H. Gauvin, P. Jollez, E. Chornet, "A diffusion model for the impregnation of lignocellulosic materials". Tappi J. 80(11), 209-219 (1997). [ Links ]
17. Kelso Jr., W.C., R.O. Gertjejansen, R.L. Hossfeld., "The Effect of Air Blockage Upon the Permeability of Wood to Liquids". Agr. Exp. Sta., University of Minnesota (1963). [ Links ]
18. Lönnberg, B., L. Robertsen, "Chemical diffusion in wood". AIChE Forest Products Symposium, 49-55 (1992). [ Links ]
19. McKibbins, S.W., "Application of diffusion theory to the washing of Kraft cooked wood chips". Tappi J., 43(10), 801-805 (1960). [ Links ]
20. Rydholm, S.A., Pulping processes. Wiley, London (1965). [ Links ]
21. Siau, J.F., Transport Processes in Wood. Springer-Verlag, New York (1984). [ Links ]
22. Solár, R., F. Kacik, Y. Melcer, "Simple semimicro method for the determination of O-acetyl groups in wood and related materials". Nordic Pulp and Paper Res. J., 2(4), 139-143 (1987). [ Links ]
23. Stone, J. E., "The Effective capillary cross-sectional area of wood as a function of pH". Tappi J., 40(7), 539-543 (1957). [ Links ]
24. Talton Jr., J.H., R.H. Cornell, "Diffusion of sodium hydroxide in wood at high pH as a function of temperature and the extent of pulping", Tappi J., 70(3), 115-118 (1987). [ Links ]
25. Zanuttini, M., V. Marzocchi, "Kinetics of alkaline deacetylation of poplar wood", Holzforschung, 51(3), 251-255, (1997). [ Links ]
26. Zanuttini, M., V. Marzocchi, M. Citroni, "Alkaline Treatment of Poplar Wood". Holz als Roh-Und Werkstoff, 57, 185-189 (1999). [ Links ]














