Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Latin American applied research
Print version ISSN 0327-0793
Lat. Am. appl. res. vol.34 no.4 Bahía Blanca Oct./Dec. 2004
Minimum gas flow rate in a countercurrent isothermal gas stripper
A. Silva, C. Castillo and J. H. Krasuk*
Departamento de Termodinámica y Fenómenos de Transporte, Universidad Simón Bolívar, Sartenejas, Baruta, Edo. Miranda, Apdo. Postal No.89000, Caracas, Venezuela
Abstract — It is presented an analytical expression for the minimum gas flowrate required for the design of an isothermal countercurrent gas stripper, when Henry's law (H > 1) applies and the gas and liquid streams are concentrated in the soluble gas of the binary gas mixture. This solution allows for a faster and more accurate result, for (GB)min, than the graphical procedure presently in use.
Key Words — Stripping. Absorption. Separation. Isothermal. Mass Transfer. Minimum Gas Flow.
* Autor to whom correspondence should be addressed
I. INTRODUCTION
One of the first steps in designing an isothermal gas stripper is to calculate the minimum gas flow rate, which satisfies the given specifications. The graphical procedure currently used when dealing with concentrated gas mixtures, consists in employing a Y, X plot, where these are molar ratios or "molal stoichiometric units" (Sherwood et al., 1975) in the gas and liquid phase, respectively. The advantage of using this type of diagrams is that the operating line is a straight line that permits, from its slope, a direct calculation of (GB)min.
The case here considered is the design of an isothermal gas stripper, for highly concentrated binary mixtures, when Henry's Law applies.
There are several gas/liquid systems of industrial interest that still follow Henry's law when the gas mixtures are concentrated in the soluble gas; some examples are hydrogen in organic liquids and petroleum cuts (Birthler et al., 1963; Chao et al., 1981; Alessi et al., 1996; Battino and Clever, 1996; Luhring and Schumpe, 1989; Herskovitz et al., 1983; King and Najjar, 1977), carbon monoxide in organic liquids (Luhring and Schumpe, 1989), carbon dioxide in water (Perry, 1963), in organic liquids (Luhring and Schumpe, 1989) and bitumens (Lal et al., 1989) and hydrogen sulphide in hydrocarbons (Lal et al., 1989). Practically all theses systems have a Henry's constant higher than unity and therefore, as demonstrated in Section II, show an equilibrium curve which is concave upward in the Y, X diagram. In a Y, X plot, when the equilibrium line is concave downward and Henry's Law applies, as is the case for H<1 as demonstrated in Section II, the solution for the inert molar ratio (LB/GB)max is straight forward:
(LB / GB)max = (Y*2 - Y1) / (X2 - X1) (1)
In the stripper design case all molar ratios in Eqn. 1 are specified except Y*2, but this is given by:
Y*2 = y*2 / (1 - y*2) (2)
where y*2 = H.x2 can be obtained from Henry's Law; consequently, Eqn. 1 allows the direct calculation of the (LB/GB)max ratio without any need of performing a Y, X plot for the isothermal stripper of concentrated gas mixtures when Henry's law applies and H<1.
The case we are dealing with is a countercurrent isothermal stripper for concentrated gas mixtures when H>1, which applies to most of the above indicated gas/liquid systems of industrial interest. In this situation the equilibrium curve is concave upwards. Fig. 8.11 in (Treybal, 1980) shows how the graphical procedure, mostly used at present to get (GB)min, is employed. This is also shown in present Fig. 3. From the point Y1, X1, which corresponds to the dilute bottom of the stripper, in the Y, X diagram, the operating line is drawn tangential to the equilibrium line. This determines the point YM, XM and the slope of this tangent gives (LB/GB)max from which (GB)min is obtained.
The present development allows the direct calculation of (GB)min, for such case, without any graphical procedure.
II. RANGE OF VALUES OF HENRY'S CONSTANT
Here it is discussed the range of values of Henry's constant to obtain an equilibrium curve with upward or downward concavity in a Y, X plot. Henry's Law applies:
y = Hx (3)
Substituting Y, X in Eqn. 3 results:
Y = X H / (1 + X (1 - H )) (4)
Since Y and X can not have negative values Eqn. 5 and Eqn. 6 must hold:
Y > 0 (5)
X > 0 (6)
Consequently, for Eqn. 5 to be true, since H is always positive,
1 + X (1 - H ) > 0 (7)
and the following expressions hold :
| H < 1 | X > 0 | (8) |
| H > 1 | 0 < X < 1/ (H -1) | (9) |
The first derivative of Eqn. 4 is:
 | (10) |
This is always positive and increasing for all values of Y and X, under Eqn. 5 and Eqn. 6; consequently the operating line tangent to the equilibrium line also has a positive slope.
The second derivative is:
 | (11) |
When H>>1, which is true for many of the gas/liquid systems indicated above, the second derivative is:
 | (12) |
When H>1, since Eqn. 7 is valid, the second derivative given by Eqn. 11 is positive, thereby giving an equilibrium curve with upward concavity. On the other hand, when H<1 the second derivative is negative and the equilibrium curve is concave downward. Because of the background given in the previous section, the development that follows deals only with the H>1 case. To our knowledge there is no analytical solution for the minimum gas flow required in the isothermal stripping of a liquid when this gives rise to a concentrated gas mixture and Henry's law applies (H>1).
III. PROCEDURE TO OBTAIN (LB/GB)max
To obtain the maximum molar ratio (LB/GB)max Eqn. 4 is equated to the operating line, which is shown in the right member of Eqn. 13:
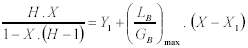 | (13) |
which after some rearrangement becomes Eqn. 14:
a X 2 + b X+ c = 0 (14)
where:
a = a.(H - 1) (15)
b = (H - 1).[Y1 - a.X1] + H - a (16)
c = a.X1 - Y1 (17)
Here above a = (LB/GB)max has been introduced.
The solution to Eqn. 14 is:
 | (18) |
Equation 18 gives the two possible points of intersection between the equilibrium and operating lines which are represented by "D" and "E" in Fig. 1. In the present case, the solution must be only one and real; therefore Eqn. 19 must be satisfied:
b2 - 4ac = 0 (19)
Equation 19 and Eqn. 18 determine point XM, where the operating line touches tangentially the equilibrium line, as shown in Fig. 1.

Substituting the coefficients "a", "b" and "c", given by Eqn. 15 through Eqn. 17, in Eqn. 19, results in another cuadratic equation in a :
da2 + ea + ¦ = 0 (20)
where
d = [(H - 1).X1 - 1]2 (21)
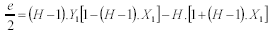 | (22) |
¦ = [(H - 1).Y1 + H]2 (23)
Finally the solution for a = (LB/GB)max is given by Eqn. 24:
 | (24) |
Since Eqn. 7 holds, the same happens with Eqn. 25:
[1 - (H - 1).X1] > 0 (25)
It is also true that:
H X1 > Y1 (26)
since X1 belongs to the dilute bottom of the tower and consequently Eqn. 26 is practically same as Eqn. 27:
H x1 > y1 (27)
This is true because the equilibrium line must be over the operating line for the stripper to function. Then the first term in the solution given by Eqn. 24 is positive and same happens with the second term that is preceded by sign "±".
Equation 24 gives (LB/GB)max only when the "±" sign preceding its second term holds. This is so because of the following reasoning.
Let us draw a generic equilibrium curve for the full range of X, positive and negative values of X (Fig. 2). Also the point Y1, X1, corresponding to the dilute bottom of the stripper tower is indicated. Eqn. 24 gives the two possible points, "A" and "B", where the tangents lines passing through Y1, X1 can touch the generic equilibrium line. Since it should hold XM >X1 it is obvious that only the steeper slope of the two straight lines, in Fig. 2, is possible and this steeper slope can only be attained if the "+" sign, before the second term in Eqn. 24, is considered as valid.
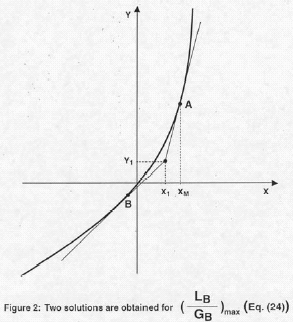
An additional demonstration to the fact that Eqn. 24, with the "+" sign before its second term, gives the correct molar ratio (LB/GB)max, is given in the Appendix.
Example 1.
The design of stripper section of Illustration 8.2 in (Treybal, 1980) is taken as an example for the application of the solution given by Eqn. 24, in this Note.
Benzene is to be stripped from a wash oil by superheated steam injection at atmospheric pressure and 122oC. The debenzolized oil of 0.005 mole fraction, in benzene, is to be cooled to 26oC and returned to an absorber. The stripper temperature is constant at 122oC. Other data are that Henry's law applies with H= 3.16. Also:
X2 = 0.119; LB = 1.787x10-3 kgmol/s;
Y1 = 0.0; X1 =0.005/ (1-0.005) = 0.00502
The application of the solution given by Eqn. 24, with the "+" sign preceding its second term, gives:

This result agrees with that given by (Treybal, 1980) with 0.24% error.
While the solution given by (Treybal, 1980) requires making a Y,X plot to obtain the tangent to the equilibrium curve, the use of the solution given by Eqn. 24 saves time and gives a result less subject to error.
Example 2
Hexane contained in a residual oil is to be stripped with nitrogen at 100oC and one atma. Content of hexane vapor at the inlet of the gas stream is 1% v/v. Other data are:
H=1.6; LB =0.021 kgmol/s; X2 = 0.517; X1 = 0.0293; Y1 = 0.0101
The application of the analytical solution (24) gives:

The result obtained from Fig. 3 by means of the graphical procedure is (GB)min = 0.0102 kgmol/s, in good agreement with the mathematical answer.
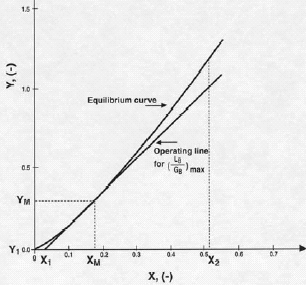
Figure 3: Graphical Procedure for Example 2
IV. CONCLUSIONS
An analytical expression was obtained which allows us to calculate the minimum gas flow rates of isothermal gas strippers when Henry's law applies (H>1) .This expression may replace the graphical procedure currently used, providing an accurate and fast method especially suited for computerized design procedures.
Appendix
Another demonstration that solution given by Eqn. 24 is only valid if the "+" sign, before its second term, is used, starts from the consideration that Eqn. A1 must hold:
XM - X 1 > 0 (A1)
The substitution of Eqn. 19 in Eqn. 18 gives:
 | (A2) |
Now the solution given by Eqn. 24 together with Eqn. 15 and Eqn. 16 are substituted in Eqn. A2 to give:
 | (A3) |
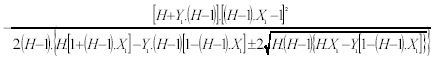 | |
XM must satisfy Eqn. A1 as a precondition for the design; the substitution of Eqn. A3 in Eqn. A1 gives Eqn. A4:
 | (A4) |
After some rearrangement Eqn. A4 becomes in unequality Eqn. A5:
 | (A5) |
Let us call Q the second term, inside the first parenthe-
sis, in the numerator of Eqn. A5.
It can be seen, because of Eqn. 26, that Q>0.; then Eqn. A6 follows:
 | (A6) |
Finally, from this expression it can be concluded that only if the "+" sign is taken in Eqn. A6 this holds.

Subscripts

Acknowledgement
We acknowledge the contribution of Professor Domingo Quiroz, Universidad Simón Bolívar, to the interpretation of results of this work.
References
1. Alessi, P., Cortesi, A., Kikic, Y. and Neau, E. "Measurements of Henry's constants for the characterization of heavy petroleum fractions" Fluid Phase Equilibria (117) 211-216 (1996). [ Links ]
2. Battino, R. and Clever, L., "The solubility of gases in liquids", Chemical Reviews 395-453, (August 1996). [ Links ]
3. Birthler, R., Karoly, J. Spitzner, M. and Zalai, J. "Solubility of hydrogen in hydrocarbons", Int. Chem. Eng. 3 597 (1963). [ Links ]
4. Chao, K, Lin, H., and Sebastian, H., "Correlation of solubility of hydrogen in hydrocarbon solvents", AIChE J, 27 138 (1981). [ Links ]
5. Frohlich, K., Tauch, E. J., Hogan, J. J. and Peer A. A. "Solubilities of gases in liquids at high pressures", Ind. Eng. Chem. 23 548-550 (1931). [ Links ]
6. Herskowitz, M., Skladman, L. and Wisniak, J., "Hydrogen solubility in organic liquids", J. Chem. Eng. Data 28 164-166 (1983). [ Links ]
7. King, M.B. and Najjar, H. "The solubilities of carbon dioxide, hydrogen sulphide and propane in some normal alkanes solvents", Chem. Eng. Sci. 32 1241-1246 (1977). [ Links ]
8. Lal, D., Mather, A. E. and Otto, F. D., "Solubility of hydrogen, hydrogen sulphide and carbon dioxide in bitumens and heavy gas oils", J. Chem. Eng. Data 28 134-137 (1989). [ Links ]
9. Luhring, P. and Schumpe, A. "Gas solubilities in organic liquids at 293.2K", J. Chem. Eng. Data, 34 250-252 (1989). [ Links ]
10. Perry, J. H., "Chemical Enginners´ Handbook" 4th Ed., McGraw-Hill, N.York, (1963). [ Links ]
11. Sherwood, T. K., Pigford, R. L., Wilke, C. R. "Mass transfer", McGraw-Hill, N. York (1975). [ Links ]
12. Treybal, R. E., "Mass transfer operations", McGraw-Hill, N. York, 3rd. Ed 287 (1980). [ Links ]














