Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Latin American applied research
versión impresa ISSN 0327-0793
Lat. Am. appl. res. v.34 n.4 Bahía Blanca oct./dic. 2004
Desorption of ethyl acetate from modified clays by supercritical carbon dioxide
G.L.V. Coelho1 and J. Pawliszyn2
1 Lab. de Processos de Separação, Departamento de Engenharia Química, Universidade Federal Rural do Rio de Janeiro, 23890-000 Seropédica, RJ, Brasil coelho@ufrrj.br,
2 Department of Chemical, University of Waterloo, Ontario, Canada N2L 3G1
Abstract ¾ The supercritical regeneration with carbon dioxide of modified and unmodified clays was experimentally studied after their use to adsorb ethyl acetate from aqueous solutions. Two quaternary amine modifiers (tetramethyl ammonium chlorine / TMA+ and hexadecyltrimethylammonium bromide/ HDTMA+) were used. The desorption of ethyl acetate adsorbed over the clays was performed with carbon dioxide at temperatures ranging from 301 K to 333 K and pressures ranging from 69.0 bar to 413.8 bar. The regeneration capacity was almost coincidental and high pressure was more favourable to regeneration. The effect of pressure and temperature was characterised under different conditions (gas, liquid and supercritical) and the supercritical has shown to be the best. A crossover effect was observed. The experimental data was fitted to a simple model, being the best results corresponding to desorption with carbon dioxide in its supercritical region.
Keywords ¾ Organo-Clay. Smectite. Adsorption. Extraction. Supercritical. Carbon Dioxide.
I. INTRODUCTION
The unreasonable discharge of various chemical materials such as organic pollutants as industrial waste has been a major environmental concern. Therefore, the use of various physicochemical and biological techniques to remove these contaminants from wastewater has been extensively researched.
Supercritical fluid extraction has become widely accepted as a replacement for classical extraction methods mainly for environmental control. Chemical engineers are also tackling issues surrounding supercritical carbon dioxide, a promising, environmentally friendly replacement for organic solvents.
For the same reasons, research regarding new adsorbents for removal of pollutants from water has also been extensively done. The study of the regeneration of these adsorbents is also very important. The regeneration of activated carbon by supercritical carbon dioxide after adsorption of ethyl acetate had already been studied experimentally (Tan and Liou, 1988). Normally, the activated carbon is used to reduce and/or to recover organic compounds in effluent streams and ethyl acetate is one of these compounds commonly emitted by the chemical industry. Clay minerals as adsorbents, especially smectite, are of particular interest because of their large specific surface areas (Park and Yeo, 1999). An alternative adsorbent is modified clay. If a clay has metal cations occupying cation-exchange sites, its surface is hydrophilic because of the water molecules in the hydration shell solvating the cations. Such a surface is not a good adsorbent for removing hydrophobic organic molecules, which have poor water solubility (Boyd et al., 1988). When the metal ions are replaced by large organic cations as those from surfactants, the nature of the clay surface is drastically altered, and turned hydrophobic or organophilic in nature. In other words, as hydrophobicity of the molecule is increased, the sorption of organics increases. The use of organo-clays as adsorbents for organic contaminants (both ionic and nonionic) from aqueous solutions has been studied. But works about desorption and regeneration using supercritical fluid conditions are scarce. In this work the regeneration of organo-clays loaded with ethyl acetate using supercritical carbon dioxide at different pressures and temperatures was studied. The clay employed was montmorillonite, and HDTM+ and TMA+ as organic modifiers were used, which gave different hydrophobic nature to the clay.
II. EXPERIMENTAL
Details about the experimental methods can be found in (Coelho et al., 2001). The adsorption of HDTMA+ and TMA+ onto montmorillonite was performed in an amount just equal to the cation-exchange capacity of the clay. In order to examine the structural difference of montmorillonite before and after the modification with a quaternary amine, the basal spacings (d001) were determined using a Powder X-ray diffractometer. In order to verify the adsorptive capacities of organically modified clays and to compare them with an unmodified clay, a batch experiment was designed.
In the adsorption experiments, the ethyl acetate solution (2.6 g/L) was pumped through the column packed with organo-clay (Fig.1). The ethyl acetate solution, at a temperature of 308 K, was passed through the packed bed and the concentration of the effluent was determined using solid phase micro-extraction - gas chromatography (SPME-GC). The column (2.7 cm3) was charged with 1.0 g organo-clay, and 1.0 cm layers of 20-30 mesh glass beds were placed above and below the organo-clay bed in order to homogenize the flow distribution and to avoid possible end effects.
For the supercritical fluid extraction (SFE) the same apparatus used for the adsorption experiment was employed (Fig.1). The experiments were performed at pressures of 69, 137.9, 275.9 and 413.8 bar and at temperatures of 301, 313 and 333 K. The flow rate was maintained at 1.0 - 2.2 ml/min. The temperature was controlled with a constant temperature bath. The CO2 stream, before reaching the column, passed through a preheated coil in the bath. The extracted ethyl acetate was collected through a stainless steel capillary restrictor (0.50-mm-i.d.) in a cold trap containing 2-propanol. The SFE extraction time profiles were obtained at various pressure and temperature combinations. In order to find out the best desorption conditions, the experiments were also performed with gaseous (G), liquid (L) CO2 (Table 1). The collected samples were analyzed by SPME-GC (Pawliszyn, 1997). SPMEwas used for extracting the ethyl acetate from the samples. A SPME fiber coated with a 30 mm thick polydimethylsiloxane (PDMS) layer was used. The desorption temperature was 150oC.
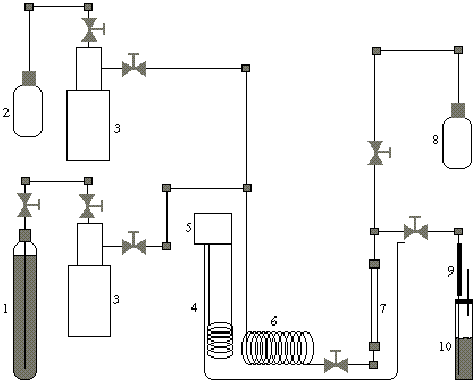
Figure 1. Schematic diagram of the apparatus used for adsorption and supercritical extraction of ethyl acetate from organoclays: 1- liquid CO2 tank; 2- ethyl acetate solution; 3- syringe pumps; 4- water bath; 5- heating; 6- preheating coil; 7- adsorption/desorption column; 8- effluent collector; 9- restrictor; 10- collector trap.
III. RESULTS AND DISCUSSION
Fig. 2 shows the results of a batch sorption experiment where it is possible to compare the adsorptive capacity of the unmodified clay (6.2 mg ethyl acetate/g-clay) with the modified clays, i.e. TMA+-clay (15.6 mg ethyl acetate/g-clay) and HDTMA+-clay (35.6 mg ethyl acetate/g-clay). It can be seen that, as expected, the nature of the clay surfaces was drastically altered. Montmorillonite saturated with (TMA+) cation showed weak sorption when compared to HDTMA+, which is more hydrophobic. In general, the more hydrophobic the group(s) associated with the quaternary ammonium entity, the greater the sorption of the sorbate (Boyd et al., 1988).
The observed different adsorption capacity led to a determination of d-spacings by X-ray diffraction analysis of the modified and unmodified clays. The adsorption of ethyl acetate was performed by montmorillonite-clay and by two different organo-clays (TMA+ and HDTMA+); the desorption was carried out with carbon dioxide at physical conditions defined by T and P, that allows the experiments to be conducted in different phases to find the best optimal operating conditions. A mass balance for the ethyl acetate compound was performed at the end of the adsorption/desorption experiments. The total volume of carbon dioxide used for the recovery of the adsorbed ethyl acetate from the organo-clay was 266 ml.
The result does not indicate a difference in regeneration efficiency between the two modified and the unmodified motmorillonite, despite the different adsorptive capacities as previously shown in Fig. 2.

Figure 2. Adsorption capacity of the modified and unmodified clays.
The effect of temperature was studied at pressures from 69 to 413.8 bar, while the effect of operating pressure on the cumulative amount of ethyl acetate desorbed was studied at temperatures from 301 to 333 K. Figs. 3, 4, 5 and 6 show the results.
The most efficient operation condition was found in the supercritical region (333 K and 413.8 bar). Under this condition, 84 % of the adsorbed ethyl acetate was recovered from HDTMA+-clay. The worst recovery efficiency result was found in the liquid phase (301 K and 69.0 bar), which yielded a recovery of only 8.6%. The result of both gas conditions used in this work showed that the recovery efficiency decreases with increasing temperature at 69.0 bar (Fig. 3). The same behavior could be noted at 137.9 bar; desorption was greatest at the lowest employed temperature above the critical temperature (Tc =304 K) (Fig.4). This effect cannot be seen at pressures of 275.9 bar and 413.8 bar as shown in (Coelho et al., 2001). Fig. 5 indicates clearly this unconventional phenomenon that is analogous to that of the solubility in a supercritical solvent, where the solubility decreases with increasing temperature at low supercritical pressure. This behavior is known as the crossover effect.
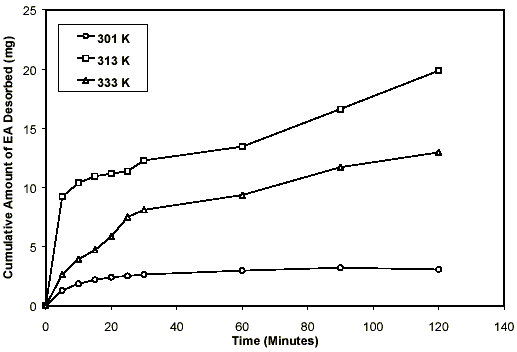
Figure 3. Effect of temperature on the desorption of ethyl acetate on HDTMA-clay at 69.0 bar.
A crossover effect has also been found for supercritical fluid desorption from activated carbon (Srinivasan et al., 1990).
The results obtained for 3 temperatures and for the pressures of 275.9 and 413.8 bar can be found in (Coelho et al., 2001).
Besides its unique solubility characteristics, a properties that add to its attractiveness. For example, even though it owns a liquid-like density over much of supercritical fluid owns certain other physicochemical

Figure 4. Effect of temperature in the desorption of ethyl acetate on HDTMA-clay at 137.9 bar.
the range of industrial interest, it exhibits gas-like transport properties of diffusivity and viscosity. Additionally, the very low surface tension of supercritical fluids allows easy penetration into microporous materials to take place. Such properties can cause different effects. Higher density may enhance the solubility, and higher viscosity may be unfavorable to the rate of diffusion.
Table 1. Ethyl acetate recovery and carbon dioxide properties at various experimental conditions.

The isothermal curves (Fig. 5), at a temperature of 301 K in the liquid phase, and at 313 K in the gas phase and going into the supercritical region, show analogous behavior, as the recovery efficiency increases at lower pressures and decreases at higher pressures. In these cases the viscosity effect seems to be dominant. The relative ethyl acetate concentration on organo-clay decreases with isothermal increases in pressure as shown in Fig. 6. The result could be explained by the dependency existing between desorption and carbon dioxide density. The increase in desorption with isothermal increases in carbon dioxide density are due to the greater solvating power of the carbon dioxide which results in faster desorption. The result confirms that ethyl acetate is both soluble in supercritical carbon dioxide and not strongly adsorbed on the modified and unmodified montmorillonite. These qualities make regeneration of all montmorillonite easier.
For modeling the desorption profiles, a simple preliminary approach has been considered (Park and Yeo, 1999; Brady et al., 1987). It was assumed that the packed bed is perfectly mixed with carbon dioxide. The mass transfer resistances are negligible in this case with equilibrium being the only contributing factor in the extraction process. A mass balance in the extraction column is expressed by
W (dq/dt) = -QC (1)
where W is the clay weight, q is the ethyl acetate concentration in the clay phase in g/g-clay, Q is the flow rate of carbon dioxide in cm3/min and C is the ethyl acetate concentration in the carbon dioxide phase in g/cm3. By using a linear partition relationship among the phases,
q = KC (2)
where K is the distribution coefficient in cm3/g-clay, the solution of Eq. (1) is given by
ln(q/ q) = (-Q/KW)t (3)
where qo is the initial concentration in the clay. A linear regression of the extraction data, ln(q/ qo) vs. t, allows the determination of the distribution coefficient and the prediction of the extraction profiles (Brady et al., 1987). Fig. 6 is plotted using these values.

Figure 5. Isothermal desorption of ethyl acetate from HDTMA-clay with supercritical carbon dioxide versus pressure.

Figure 6. Predicted vs experimental extraction profiles of ethyl acetate at 333 K with supercritical carbon dioxide. Solid lines represent the data obtained by Eq.(3), and symbols represent the experimental data
IV. CONCLUSION
The supercritical regeneration with carbon dioxide of modified and unmodified clays was experimentally studied. Two quaternary amine modifiers (HDTMA+ and TMA+) were used. The effect of pressure and temperature was characterized under different conditions (gas, liquid and supercritical) and the supercritical was shown to be the best. A crossover effect for desorption was observed, i.e. desorption decreases with increasing temperature. The roles of the density and viscosity were observed to be determinants of the optimal conditions. The most active form of montmorillonite for ethyl acetate adsorption was the clay that had been modified with HDTMA+.
REFERENCES
1. Boyd, S.A., S. Shaobai, J.F. Lee and M.M. Mortland, "Pentachlorophenol Sorption by Organo-Clays," Clays and Clays Minerals, 36, 125-130 (1988). [ Links ]
2. Brady, B.O., C.P.C. Kao, K. M. Dooley, F.C. Knopf and R.P. Gambrell, "Supercritical Extraction of Toxic Organics from Soils," Ind. Eng. Chem. Res., 26, 261-268, (1987). [ Links ]
3. Coelho, G.L.V., F. Augusto and J. Pawliszyn, "Desorption of Ethyl Acetate from Adsorbent Surface (Organoclays) by Supercritical Carbon Dioxide," Ind. Eng. Chem. Res., 40, 364-368, (2001). [ Links ]
4. Park, S.J. and S.D. Yeo, "Supercritical Extraction of Phenols from Organically Modified Smectite," Sep. Sci. Technol., 34, 101-113, (1999). [ Links ]
5. Pawliszyn, J., Solid Phase Microextraction - Theory and Practice, Wiley-VCH, Inc., New York, (1997). [ Links ]
6. Srinivasan, M.P. J.M. Smith and B.J. McCoy, "Supercritical Fluid Desorption from Activated Carbon," Chem. Eng. Sci., 45, 1885-1889, (1990). [ Links ]
7. Tan, C.S. and D.C. Liou, "Desorption of Ethyl Acetate from Activated Carbon by Supercritical Carbon Dioxide," Ind. Eng. Chem. Res., 27, 988-991, (1988). [ Links ]














