Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Latin American applied research
versão impressa ISSN 0327-0793
Lat. Am. appl. res. v.35 n.1 Bahía Blanca jan./mar. 2005
Preliminary evaluation of TEDMA/HEMA + HAP composites as bone substitutes and drug controlled delivery matrixes
G. Fuentes, A. Lara, E. Peón and M. Torres
Biomaterials Center, University of Havana, P. O. Box 6130, Postal Code 10600, Havana, Cuba.
gastonfe@biomat.uh.cu
Abstract ¾ Copolymeric composites of tetraethylenglycol dimethacrylate and 2-hydroxyethyl methacrylate with hydroxyapatite load were studied. It was demonstrated that doesn't exist a tendency to justify the behavior of the work and setting times of the studied materials cause probably by inhibitor absence. Similar values of enthalpy reactions were obtained for all the compositions according to the literature reports. A strong dependence of the composition exists in case of mechanical properties, diminishing the compression strength with the increase of the hydrofilicity. In a same way, the little swelling of the samples is demonstrated and therefore, the non participation of the diffusion like delivery mechanism. It was calculated the quantity of liberated drug which influences notably in the mechanical properties of the material and some parameters that suggest it mechanisms type dissolution-diffusion and/or migration to guide the drug delivery.
Keywords ¾ Composites. Hydroxyapatite. Acrylic Monomers. Drug Delivery Systems. Mechanical Properties.
I. INTRODUCTION
The hydroxyapatite, HAP, is a fundamental mineral component of the human bone. The preparation of calcium phosphates cements that form in situ HAP have been studied as a possible solution to bone traumatologies that have been increased in our days. Composites of PMMA with HAP or dense and porous blocks of calcium phosphates with diverse morphologies have enlarged too (Arcís, 1998 and Bowen et al., 1978). It was demonstrated that an increase in the volume fraction of hydroxyapatite from 10 to 50 % produced an increment in the compression strength of the composite (Guild and Bonfield, 1993). This fact enlarges the original properties of the hydroxyapatite in order to apply in zones of high mechanical charges (Arcís, 1998).
The human skeleton constitutes a novel element in the evolutionary development for its regeneration capacity and the physiologic evolution of tissues formed in embryonic state. Novel synthetic and natural materials have been elaborated for improving this regeneration and restoration capacity. The biomaterials before mentioned can be biodegradable and not biodegradable, and they have advantages and disadvantages (Bowen et al., 1978).
The ideal material for the bone substitution should imitate to the natural bone tissue that replaces it in size, forms, consistency and operation. It should not cause infections neither to cause foreign body response. And should be tolerated permanently by the receiving organism, in other words, it should be biocompatible. As main inorganic component of the skeleton and teeth of most of the vertebrates, the hydroxyapatite is one of the few materials that doesn't form a reaction when being implanted in the live bone tissue. That is the reason of the great interest wakened up by the ceramic and other materials based on them as implant object (Bowen, 1979).
There are a large variety of degradable polymers available for use in surgery which are generally based on blends and copolymers of poly(L-lactide) and poly(L-glycolide) (Knowles, 1993). Different poly(2-hydroxyethyl methacrylate)/polycaprolactone hydrogel composite systems reinforced with polyethylene therephtalate fibres have been investigated for potential use as invertebral disc prostheses (Ambrosio et al., 1998).
Also, copolymers of 2,3-dihydroxypropyl methacrylate and 2-hydroxyethyl methacrylate have been studied as a interconnected network in order to use as possible implant material (Gates et al., 2003a and Gates et al. 2003 b). Other polymers was filled with HAP through a method based on spontaneous precipitation of HAP in aqueous suspensions of sulphonated polysulphone polymers particles (Spanos et al., 2002).
Another group of researchers have been proposed investigations about composites HAP-PMMA. They determine wether the incorporation of HAP in a PMMA matrix would enhance the biological properties of osteoblast response as compared to PMMA alone (Moursi et al., 2002).
In this work the results of a preliminary study of a self-cured composite based on a copolymeric mixture of tetraethylenglycol dimethacrylate (TEDMA) and 2hydroxyethyl methacrylate (HEMA) loaded with HAP are presented. It tries to prove the influence of the copolymeric composition in the work and setting time and enthalpy polymerization. Also it was determined the effect over mechanical properties and the controlled delivery capacity of the system as drug support matrix.
II. METHODS
The HAP was obtained for synthetic way in our laboratory from a reaction with Ca(OH)2 and H3PO4 checked to IR, DRX and TG. The molar ratio Ca/P was 1.66. The monomers (TEDMA and HEMA), the initiator (PBO, benzoyl peroxide) and the amine (DMpT, N, N-dimethyl-p-toluidine) used in the composite preparation came from FLUKA AG and MERCK. All the used materials had purity bigger than 95% and were used without previous purification.
The composites were prepared varying the composition in weight from 30 to 70 % of the monomers as shown in Table 1. To the monomeric mixtures are added PBO (2 %) and 50% of HAP in case of the base paste preparation and DMpT (1.5 %) and 50% of HAP in case of catalytic (Bowen, 1979). The reacting mixture was prepared by mixing similar quantities of the base and catalytic pastes (Fig. 1), assuming 0.6 grams approximately of mass from the available molds for the mechanical properties (EN ISO 604, 1993) of dental obturants. The samples prepared by these standards were used for all the studies (ISO 4049, 2000).
Table 1. Monomeric mixtures composition (50% HAP)
The drug (sodium cefuroxime) was incorporated to evaluate the delivery ability and the effect of their inclusion on the mechanical properties. The swelling was calculated by the gravimetric method at 37oC in distilled water. The samples were weigh and evaluating according to the equation  (Escobar et al., 2000b). The calorimetric measures were carried out in a METTLER TA 3000 DSC 30 under air dynamic atmosphere, in a isostatic regimen at 37oC. The mechanical properties were carried out in universal machine TIRAtest 2300 enabled with a cell of 10 kN.
(Escobar et al., 2000b). The calorimetric measures were carried out in a METTLER TA 3000 DSC 30 under air dynamic atmosphere, in a isostatic regimen at 37oC. The mechanical properties were carried out in universal machine TIRAtest 2300 enabled with a cell of 10 kN.
The drug delivery studies were carried out in MLW UH4 thermostat with temperature range of 0-300oC and capacity of 5 L of recycling liquid. The liberated drug was determined by means of spectrophotometric measures in a SECOMAM UV-visible, model S 1000 with band wide of 2 nm, work range of 200-1000 nm, precision of ± 0.3 nm and reproducibility of ± 0.1 nm.

Figure 1. Preparation scheme. If X=PBO, Y=Base. If X=DMpT, Y= Catalytic
III. RESULTS AND DISCUSSION
A. Thermo Analytical Characterization
In thermo analytical evaluation of the studied composites it is observed an oscillation of the values approximately between 40 and 60 kJ/mol as observes in Table 2. The immense majority of the acrylates has a value of DHr that oscillates between those values obtained in our studies (Rosado, 2000; Coover and McIntire, 1989 and Schoenberg, 1985).
Table 2. DHr of the composites.

Perhaps the presence of hydroxyapatite in a radical copolymerization works as capable agent to adsorb a part of heat generated by the copolymerization process, that would make the interpretation of this phenomena more complex what is presented in the reality, although in this case the presence of HAP do not represent any influence over the polymeric matrix (García, 2000).
The working (tw) and setting (ts) times were calculated according to the norm ISO 4049 (1988) with similar behavior that in enthalpy case.
In other words, the working time oscillated between 0.18 y 1.17 min (1.5 min as minimum according ISO 4049, 1988). No one formulation fills this requirement perhaps cause the inhibitor absence and the setting time oscillated between 0.42 y 1.42 min (< 10 min), in this case according with reported in the international standards.
From this behavior it is not inferred that we can assume a dependent tendency of the composition for the values of work and setting times, which being explained by the same causes of the enthalpy (Bowen, 1978 and García, 2000).
The "mesophase" theory and the demonstrated importance of the treatment of the filler surfaces to 5. 25 achieve a better adhesion from the inorganic loads to the polymeric matrix (García, 2001, Stupp and Ciegler, 4. 50 1992) could also be attributed. The superficial treatment with silane improves the adherence from filler particles to organic matrixes because the SiOH groups allows a better connection with the filler inorganic structure from Mt ( mg ) 3. 75 3. 00 2. 25 1. 50 the Si and connection type hydrogen bridge with polymeric molecules achieving a better absorption and an 0. 75 improvement of the composite properties (Veranes et al., 2002).
B. Mechanical Characterization of Composites
The compression strength for the TEDMA-HEMA polymer materials indicated a trend of lower values with increasing proportions of HEMA as shown in Fig 2 (Jones and Rizkalla, 1996 and Moursi et al., 2002). Besides, in case of drug addition to the matrix the compression strength diminishes related with matrix without drug maybe cause by interactions between polymers and drug.
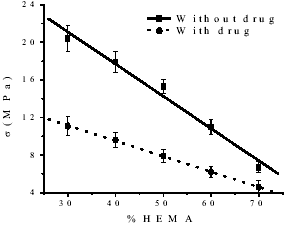
Figure 2. Variation of V (MPa) vs. HEMA content.
It is remarkable the difference between both experiments, which could be due to the drug dissolution in the matrix and the increase of the absorption capacity caused by the mentioned growth of the hydrophilic character of the matrix. This fact can be certifying by studies of diffusion mechanism as shown in Table 3.
C. Kinetic Study of Drug Delivery Ability
The cefuroxime delivery was carried out to (37 ± 1)oC, and the obtained profiles are shown in the Fig. 3. The sample with major amount of hydrophilic monomer is the most capable to liberate, as we expected, cause the higher percentage of near chains to water allows a better interaction between solution and matrix (Zhang et al., 1994).
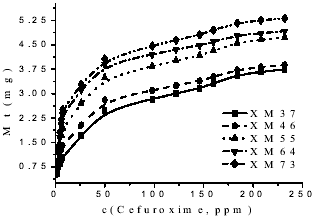
Figure 3. Release profiles of the studied samples.
It is to point out that the studied matrixes do not swell as they demonstrate it the comparison between the initial and final masses of the matrixes (Table 3) like hydrophilic classic matrixes (Solá, 1998). The swelling percent was calculated by the equation  . All the composites was washing with destilled water three times and dried for 24 hours to ambient.
. All the composites was washing with destilled water three times and dried for 24 hours to ambient.
Also, from Fig. 3, we can obtain the data where at the end of all process liberates between 7 and 15 % of the drug quantity which were doped the initial samples.
Table 3. Comparison between initial and final masses of studied samples and swelling percent

It is important to notice that the swelling percent of the samples increases when growing the hydrophilic monomer percent, according to literature report (Escobar et al., 2000b; Agüero et al., 2000 and Gates et al., 2003b). Perhaps, we can assume that the hydroxyapatite presence does not provoke any change in classical behavior of the composites.
As we can see the absolute value of swelling is smaller if we comparing with other acrylic monomers systems. Maybe this fact justifies the values of diffusion coefficients, discuses in Table 3 (Escobar et al., 2000a). This can lead to question the diffusion like delivery mechanism of the samples.
So that, and still when the samples in a first stage fulfill the Higuchi treatment (Higuchi and Higuchi, 1960), in the second stage doesn't happen the same, it doesn't adjust none of the traditional models. From the fact that previously enunciates about question of the diffusion as mechanism guideline of the release process, we show this approach in Fig. 4 and 5.

Figure 4. Higuchi treatment for the first stage of the release studies.

Figure 5. Higuchi treatment for the second stage of the release studies.
For this reason, we decided to carry out a mechanisms search that adjusted to these release results and we found the treatment proposed by Baker (Baker and Lonsdale, 1974). Applying this adjustment to the original data was obtained the results are shown in the Fig. 6. It was plotted  with respect to the root of time inverse, and very appropriate adjustments were obtained for this treatment comparing them with the coefficient of critical correlation for 12 (f=m-2) degrees of freedom. The Table 4 shows the statistical results from the adjustments to the Baker treatment and it can be observed they correlate appropriately with the position proposed for a confidence level a=0.001 enunciated in the previous paragraph.
with respect to the root of time inverse, and very appropriate adjustments were obtained for this treatment comparing them with the coefficient of critical correlation for 12 (f=m-2) degrees of freedom. The Table 4 shows the statistical results from the adjustments to the Baker treatment and it can be observed they correlate appropriately with the position proposed for a confidence level a=0.001 enunciated in the previous paragraph.
Table 4. Adjustment results to Baker treatment.
rcrit(0.001, 12) = 0.780


Figure 6. Baker treatment for the studied samples
It is important to show that when increasing the hydrophilic characteristics of composites, this enlarges the adjustment slopes of the samples, giving a better delivery ratio.
The diffusion coefficient of the all studied composites is calculated from the equation  , and results are shown Table 5 (Baker and Lonsdale, 1974 and Richards, 1986). As it is observed, the diffusion coefficient is extremely small respect other systems with HEMA (Solá, 1998 and Gates et al., 2003a).
, and results are shown Table 5 (Baker and Lonsdale, 1974 and Richards, 1986). As it is observed, the diffusion coefficient is extremely small respect other systems with HEMA (Solá, 1998 and Gates et al., 2003a).
Table 5. Diffusion coefficients of the studied samples

The first member of Baker's treatment is very connected with Fick's law of diffusion, and that's the reason cause we say that the mechanism is part of diffusion, and part of migration-dissolution.
This last case related with second term of Baker's equation, taking reference to migration of drug through the polymeric network and dissolution in phosphate buffer in order to leave the matrix from diffusion way.
Noted one more time, another reason to justify the direct influence of the composition over delivery ratio. Baker suggests for these kinds of devices (insoluble non-porous matrixes in water), a mechanism of migration or dissolution-diffusion.
IV. CONCLUSIONS
Five composite formulations with work times oscillated between 0.18 and 1.17 min and setting times between the 0.43 and 1.42 min were obtained. In case of enthalpy reaction, they oscillated between 40 and 60 kJ/mol, agree with that reported in literature for the acrylates. In both cases, any tendency explain the behavior of the samples in the measure of this properties.
When the HEMA percents increased the samples compression strength diminished, almost in lineal form, because the augment of the hydrophilic monomer make the samples less cross linked. The drug inclusion reduces the sample values of mechanical properties because the drug solubility capture hydrophilic monomer to react and augment the occlusion sites and holes inside the matrix diminishes the strong of bonding.
When we increases the HEMA percent the delivery ability enlarges, although the samples swelling was insignificant for the period in study (250 h), drug delivery between 7-15 % was achieved. The treatments of Higuchi and Baker were evaluated to discuss the drug delivery mechanisms of the samples.
It is analyzed the relevance of the adjustment parameters statistically and the diffusion coefficients are calculated to certify the migration and the dissolution-diffusion like the mechanisms that control the delivery ability from these matrixes from the obtained results of the treatment before mentioned.
REFERENCES
1. Agüero Luztonó L., D. Zaldívar Silva and J.L. Escobar Ivirico; "Cephalexine liberation from poly (acrylamide-co-methacrylic acid) hydrogels", Biomecánica, 8(1), 58-62 (2000). [ Links ]
2. Ambrosio L., R. de Santis and L. Nicolas; "Composite hydrogels for implants", Proc. Instn. Mech. Engrs, 212(H), 93-99 (1998). [ Links ]
3. Arcis Soriano R.W.; Preparation and characterization of Bis-GMA/HA composites, MSc Thesis, Institute of Material Sciences of Barcelone, (1998). [ Links ]
4. Baker R.W. and H.K. Londsdale; Controlled release of biologically active agents, Plenum Press, New York, 15-71 (1974). [ Links ]
5. Bowen R.L., H.H. Chandler, H.O. Wyckoff Jr. and D.N. Misra; "Metal-filled resin composites. Part II", J. Dent. Res., 57(2), 213-220 (1978). [ Links ]
6. Bowen R.L.; "Adhesive bonding of various materials to hard tooth tissues. XVIII: Synthesis of a polyfuntional surface-active comonomer", J. Dent. Res., 58(3), 1101-1107 (1979). [ Links ]
7. Coover H.W. and J.M. McIntire; "Acrylic and methacrylic ester polymers", Encyclopedic of Polymer Sciences and Engineering, John Wiley and Sons Eds, New York, 1, 299-305 (1989). [ Links ]
8. Escobar Ivirico J.L., L. Agüero Luztonó, D. Zaldívar Silva and E. Ramírez; "In vitro swelling study and biocompatibility preliminary evaluation of poly (acrylamide-co-methacrylic acid) hydrogels", Biomecánica, 8(1), 54-57 (2000a). [ Links ]
9. Escobar Ivirico J.L., D. Zaldívar Silva, G. Fuentes Estévez and L. Agüero Luztonó; "Synthesis of poly (acrylamide-co-methacrylic acid) copolymer. Influence of composition over physico-chemical properties", Revista CNIC: Ciencias Químicas, 31(3), 143-148 (2000b). [ Links ]
10. EN ISO 604:1996; "Determination of stress properties", Spanish version of EN ISO 604:1993, AENOR (1997). [ Links ]
11. García Carrodeguas R.; Calcium phosphates bone cements, PhD Thesis, BIOMAT (2000). [ Links ]
12. García Carrodeguas R.; "Dental composites: Effects of the interphase and other factors over durability", Revista de Plásticos Modernos, 535, 54-63 (2001). [ Links ]
13. Gates G., J.P. Harmon, J. Ors and P. Benz; "2,3-Dihydroxypropyl methacrylate and 2-hydroxyethyl methacrylate hydrogels: gel structure and transport properties", Polymer, 44, 215-222 (2003a). [ Links ]
14. Gates G., J.P. Harmon, J. Ors and P. Benz; "Intra and intermolecular relaxations 2,3-dihydroxypropyl methacrylate and 2-hydroxyethyl methacrylate hydrogels", Polymer, 44, 207-214 (2003b). [ Links ]
15. Guild F.J. and W. Bonfield; "Predictive modelling of HAP-polyethylene composite", Biomaterials, 14 (13), 985-993 (1993). [ Links ]
16. Higuchi W.I. and T. Higuchi; "Theoretical analysis of diffusional movement through heterogeneous barriers", J. Am. Pharm. Assoc., Sci. Eds., 49, 598-606 (1960). [ Links ]
17. ISO 4049; Dentistry - Resin-based filling materials, 10th edition (1988). [ Links ]
18. ISO 4049 2000 (E); Dentistry - Polymer-based filling restorative and luting materials, (2000). [ Links ]
19. Jones D.W. and A.S. Rizkalla; "Characterization of experimental composites biomaterials", Journal of Biomedical Materials Research (Applied Biomaterials), 33, 89-100 (1996). [ Links ]
20. Knowles J.C.; "Development of a natural degradable polymer of orthopaedic use", J. Med. Eng & Techn., 17 (4), 129-137 (1993). [ Links ]
21. Moursi A., A. Winnard, P. Winnard, J. Lannutti and R. Seghi; "Enhanced osteoblast response to a polymethyl methacrylate-hydroxyapatite composite", Biomaterials, 23, 133-144 (2002). [ Links ]
22. Richards J.H.; "The role of polymer permeability in the control of drug release", Chapter 6 in: Polymer Permeability edited by J.Comyn, Elsevier Applied Science Publishers, London (1986). [ Links ]
23. Rosado E., Complementary studies of some alquil cyanoacrylates with adhesives properties, Chemistry Graduated Thesis, BIOMAT (2000). [ Links ]
24. Schoenberg A.E., "Adhesives and sealants", Engineering Materials Handbook, 3, 126 (1985). [ Links ]
25. Solá Pérez J.; Composition influence of acrilamide/2-hydroxyethyl methacrylate copolymer over swelling process and ciprofloxacine release, Chemistry Graduated Thesis, BIOMAT (1998). [ Links ]
26. Spanos N., V. Deimede and P.G. Koutsoukos; "Functionalization of synthetic polymers for potential use as biomaterials: selective growth of hydroxyapatite on sulphonated polysulphone", Biomaterials, 23, 947-953 (2002). [ Links ]
27. Stupp S.I. and G.W. Ciegler; "Organoapatites: Materials for artificial bone. I. Synthesis and microstructure", Journal of Biomedical Materials Research, 26, 169-183 (1992). [ Links ]
28. Veranes Y., D. Correa, R. Krael, R. Alvarez and M. de la Nuez, "Obtención de resinas dentales fotopolimerizables", Memorias XVII Conferencia de Química de la Universidad de Oriente, QF-P7, Stgo de Cuba, Cuba, ISBN:959-207-083-0 (2002). [ Links ]
29. Zhang X., U.P. Wyss, D. Pichora and M.F.A. Goosen; "Biodegradable controlled antibiotic release devices for osteomyelitis: optimization of release properties", J. Pharm. Pharmacol., 46, 718-724 (1994). [ Links ]














