Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Latin American applied research
versión impresa ISSN 0327-0793
Lat. Am. appl. res. v.35 n.1 Bahía Blanca ene./mar. 2005
Hydrogenation of citral on Pt and PtSn supported on activated carbon felts (ACF)
I. M. J. Vilella, S. R. de Miguel and O. A. Scelza
Instituto de Investigaciones en Catálisis y Petroquímica (INCAPE), Facultad de Ingeniería Química
(Universidad Nacional del Litoral)-CONICET, S. del Estero 2654, (3000) Santa Fe,
oascelza@fiqus.unl.edu.ar.
Abstract ¾ In this paper a study about the effect of the Sn addition to Pt supported on activated carbon felts (ACF) on the catalytic behaviour in citral hydrogenation is reported. Catalysts with different Sn contents were prepared by successive impregnation of ACF previously purified by a treatment with HCl, HNO3 and HF and a subsequent thermal treatment with H2. PtSn/ACF catalysts with tin contents between 1 and 3 wt% were tested in the citral hydrogenation and characterized by using test reactions of the metallic phase (cyclohexane dehydrogenation and cyclopentane hydrogenolysis), H2 chemisorption and XPS techniques. Results of the citral hydrogenation show that the modification of the Sn content leads to an important change in the selectivity in citral hydrogenation. Thus, the Pt/ACF catalyst has an important selectivity to isopulegol, citronellal and acetals. When Sn is added to Pt/ACF, the nerol and geraniol formation is clearly enhanced. Taking into account both the catalytic results and those of the characterization techniques, a probable structure of the catalytic surface is proposed.
Keywords ¾ Activated Carbon Felts. Citral Hydrogenation. Metallic Catalyst Characterization.
I. INTRODUCTION
Citral is the main component of the lemongrass oil. The citral hydrogenation could lead to compounds of high values like isopulegol (by cyclization of citronellal). This is a very important intermediary in the production of menthol, which has several uses in the pharmaceutical industry, cosmetics, etc. Moreover, the hydrogenation of citral could produce unsaturated alcohols, which have important applications in the synthesis of perfumes, flavours and pharmaceutical products. The use of selective catalysts could avoid the high cost of the separation processes. In the last years the application of solid catalytic formulations in this reaction has been increased. Several metallic systems supported on different materials have been explored. Thus, Ni supported on SiO2 leads to citronellal and citronellol as main products (Salmi et al., 2000). The use of Os, Ru and Co would favour the production of nerol and geraniol (Galvagno et al., 1993; Singh and Vannice, 2001).
According to Didillon et al. (1993) the controlled addition of Sn, Pb or Ge to Rh/SiO2 (by using the controlled surface reaction procedure) makes possible the modification of the selectivity to different products. Thus, for a low surface coverage of Rh by Sn, Pb or Ge, the catalysts are selective for the hydrogenation of the -C=C- bonds, but for higher coverage values the catalysts are selective for the hydrogenation of the carbonyl group. The use of granular activated carbon as a support of metals was studied by Neri et al. (1994, 2002). They found that the Sn addition to Pt/C enhances the selectivity to unsaturated alcohols. It must be indicated that several authors have reported an important influence of the support on the activity and selectivity in the hydrogenation of unsaturated a-b aldehydes (Homs et al., 2001; Vannice and Sen, 1989; Malathi et al., 2001).
The use of new carbonaceous materials such as fibers, clothes and felts of activated carbons as a support of metals has been scarcely explored (Macías Pérez et al., 1997; Kogan et al., 1993; de Miguel et al., 2002). These materials have several advantages over the traditional granular carbon, mainly due to a more uniform porous structure, the high permeability and mechanical resistance. In this paper a study on Pt and PtSn catalysts supported on an activated carbon felt (ACF) is reported. The supported metallic catalysts were tested in the hydrogenation of citral in liquid phase, and characterized by using test reactions of the metallic phase, H2 chemisorption and X-ray photoelectron spectroscopy (XPS).
II. EXPERIMENTAL
A phenolic derived activated carbon felt ACN 210 15 AC (from GUN EI Chemical Industry Co. Ltd) with a SBET= 1661 m2 g-1, pore volume= 0.59 cm3 g-1) was used as a support. The support (initial content of impurities = 1.5 wt%; Mg: 0.06, Ca: 0.30, K: 0.435, Si: 0.165, P: 0.075, Cr: 0.024, Al: 0.037, S: 0.05, Ti: 0.018 and Cl: 0.165 wt%; Fe+Sn+Zn: balance) was purified by successive treatment with aqueous solutions of HCl, HNO3 and HF (10 wt%) and submitted to a thermal treatment with flowing H2 at 1123 K. The successive treatment with acids was used to eliminate the inorganic impurities, while the treatment with H2 was used for the elimination of sulphur, which is a poison of Pt (Torres et al., 1997). After these treatments the content of impurities decreased up to 0.28 wt%, according to the EDX results.
Pt(5 wt%)/ACF was prepared by impregnation of the support with an aqueous solution of H2PtCl6. Then, the catalyst precursor was dried at 393 K, overnight. The amount of Pt in the impregnating solution was such as to obtain a final Pt loading on the catalysts of 5 wt%. Bimetallic PtSn catalysts with the same Pt content (5 wt%) but with different Sn contents (PtSn(1 wt%)/ACF, PtSn(1.6 wt%)/ACF and PtSn(3 wt%)/ACF) were prepared by impregnation of the monometallic catalyst precursor with a hydrochloric solution of SnCl2. The Sn amount in the impregnating solution was such as to obtain the desired Sn loadings in the catalysts. Impregnations were carried out at 298 K during 2 h, by using a volume of the impregnation solution and the ACF weight ratio of 60 ml g-1 and a stirring rate of 1400 rpm. After Sn addition to the Pt catalyst precursor, the Pt content was not modified according to the chemical analysis results.
The hydrogenation of citral was carried out in a discontinuous batch reactor with a device for sampling the reaction products. Thus, small amounts of reaction samples were withdrawn from the reactor at different reaction times. Reaction was carried out at 343 K and at atmospheric pressure. 2-propanol (from Merck), previously saturated with H2, was used as a solvent. The volume of solvent, the citral amount (Sigma, 61% cis and 36% trans) and the weight of catalyst used in the experiments were: 30 ml, 0.30 ml, and 0.30 g, respectively. Previous to the reaction, catalysts were reduced "in situ" at 623 K under flowing H2 during 3 h. The reaction mixture was stirred at 1400 rpm and the products were analysed in a GC chromatographic system by using a Supelcowax 10M column coupled with a FID detector.
Catalysts were characterized by test reactions of the metallic phase (cyclohexane-CH- dehydrogenation, CHD, and cyclopentane-CP-hydrogenolysis, CPH), H2 chemisorption and XPS. Test reactions were carried out in a flow reactor. CHD was carried out at 573 K by using a H2/CH molar ratio= 26. CPH was performed at 623 K by using a H2/CP molar ratio= 29. Previous to the reaction, catalysts were reduced during 3 h under flowing H2 at 573 or 623 K. H2 chemisorption measurements were carried out in a discontinuous equipment. Samples were previously reduced at 623 K under flowing H2 for 3 h and then evacuated (5 10-5 torr) and cooling down to room temperature. The adsorption isotherm was performed at room temperature between 0 and 100 torr. XPS measurements were carried out in a VG-Microtech Multilab spectrometer. This equipment operates with an energy power of 50 eV (radiation MgKa, hn=1253.6 eV). The pressure of the analysis chamber was maintained at 4 10-10 torr. Samples were previously reduced with H2 at 623 K during 3 h. BE of the signals were referred to the C1s peak at 284.9 eV. Peak areas were estimated by approximation with Lorentzian-Gaussian curves.
III. RESULTS AND DISCUSSION
Fig.1 shows the scheme of the hydrogenation of citral (Galvagno et al., 1993):
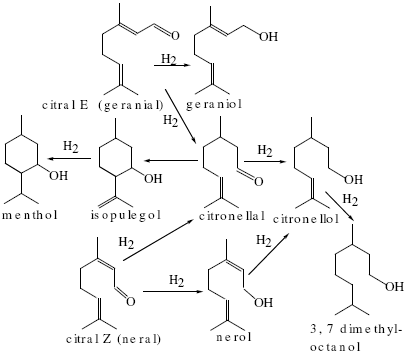
Fig.1: Scheme of citral hydrogenation .
As it can be seen in Fig.1, citral has three groups able to be hydrogenated: one carbonyl group, one C=C double bond conjugated with the carbonyl group and one -C=C- isolated double bound.
Table 1:Percentageofcitral conversion/g Ptattwo reaction times (RT: 0.5 and 4 h) for catalysts supported on ACF with different Sn contents.
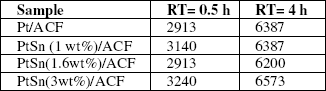
Table 1 shows the results of the catalytic activity in citral hydrogenation (expressed as the percentage of citral converted to different products per g Pt) at two reaction times (RT: 0.5 h and 4 h).
It can be observed from Table 1 that the catalytic activity is not modified when the tin amount added to Pt (5 wt%)/ACF catalyst increases. Besides, by comparing these results with those of Vilella et al. (2003) obtained on PtSn catalysts supported on granular carbon (C), it can be concluded that the latter catalysts displayed a lower activity than those supported on ACF. For example, the conversion of citral/g Pt for PtSn(2 wt%)/C at 4 h of reaction time was 5533%/g Pt.
Table 2 shows the selectivities values in the citral hydrogenation for PtSn/ACF catalysts with different tin contents. Selectivity was defined as the percentage of a given product with respect to all the products. The selectivity values in Table 2 were measured at citral conversion of 70%. At higher citral conversions the selectivity to nerol + geraniol slightly decreases and the selectiviy to citronellol and 3,7 dimethyloctanol increases for all catalysts.
Table 2: Selectivity (%) to different products in citral hydrogenation on PtSn/ACF catalysts with different Sn contents (measured at citral conversion of 70%).

*: Menthol + 3,7 dimethyloctanol
Table 2 clearly shows a modification of the distribution of the reaction products when the Sn content increases. Thus, it is observed for the monometallic catalyst a low formation of unsaturated alcohols (nerol, geraniol and citronellol), an important production of isopulegol (which is the main product), and an appreciable formation of citronellal and acetals.
When the Sn content in the bimetallic catalysts increases, the selectivity to nerol + geraniol strongly increases, reaching values higher than 70% for the catalyst with the highest tin content. Besides, the formation of isopulegol appears to be negatively affected by the tin addition to Pt/ACF. The acetals and citronellal formation also decreases, while the citronellol production is slightly lower for the bimetallic catalysts with respect to the values obtained for the monometallic sample. It must be indicated that the acetals are produced by reaction between citral and citronellal with the alcohol used as a solvent. This reaction is catalyzed by acidic centers of the support and can be partially inhibited by using branched alcohols such as 2-propanol (Tiainen, 1998), because the presence of an alkyl group in the vicinity of the OH group of the alcohol would produce an steric restriction for the reaction. The isopulegol formation is also catalyzed by acidic centers of the support. From the results of Table 2, it can be observed the parallel decrease of the acetals and isopulegol formation when the Sn content increases. Hence, this behaviour can be related to a poisoning effect of Sn on the acidic centers of the ACF. It must be indicated that other authors found a poisoning effect of Sn on the acid sites of alumina (Baronetti et al., 1986). Table 2 shows that the selectiviy to nerol + geraniol is clearly enhanced by the Sn addition to Pt/ACF, which means that the carbonyl group is preferentially hydrogenated with respect to the hydrogenation of double -C=C- bonds. Homs et al., (2001) reported that the addition of Sn to Pt increases the selectivity to the hydrogenation of carbonyl group as a consequence of two effects: i) the oxygen of the carbonyl group is activated or polarized by the action of ionic Sn species present on the metallic surface, and ii) the dilution of Pt atoms by Sn would inhibit the hydrogenation of the -C=C- bonds and the adsorption of the unsaturated alcohols, thus avoiding its isomerization to the saturated aldehydes and the subsequent hydrogenation to saturated alcohols. It must be indicated that several authors reported a positive effect of the residual chloride on the selectivity to unsaturated alcohols (Homs et al., 2001; Bachiller Baeza et al., 2002). The residual chloride contents (after reduction in H2 at 623 K) for all catalysts reported in this paper were about 0.35-0.50 wt% for all the catalyst series. Taking into account the low selectivity to nerol + geraniol found in the monometallic catalyst, it can be concluded that the effect of the residual chloride appears not to be an important factor on the selectivity in our case.
In order to obtain a relationship between the nature of the metallic phase with the catalytic behaviour, test reactions of the metallic phase, X-ray photoelectron spectroscopy (XPS) and H2 chemisorption experiments were carried out on the different catalysts supported on ACF.
Table 3 shows the results of the test reactions: cyclohexane (CH) dehydrogenation (initial reaction rate, RCH, and activation energy, ECH) and cyclopentane (CP) hydrogenolysis (initial conversion, X0CP).
It is observed in Table 3 a low value of the initial cyclohexane dehydrogenation rate (which can be considered as a measurement of the Pt dispersion) for the monometallic catalyst (Pt/ACF). This value is clearly lower than that found for catalysts with a lower Pt loading (Torres et al.,1997).
Table 3: Initial reaction rate (RCH, mol CH/h g Pt) and activation energy (ECH, kcal/mol) in CH dehydrogenation, and values of the initial conversion (X0CP, %) in CP hydrogenolysis.

Hence, the low CHD rate observed for the Pt (5 wt%)/ACF could be related to a lower Pt dispersion. Besides, Table 3 displays that both the initial reaction rate in CH dehydrogenation and the initial conversion in the CP hydrogenolysis show a low diminution as the Sn content increases. The CH dehydrogenation is a structure-insentive reaction and any change in the reaction rate lower than one order of magnitude must be considered as a small modification of the dehydrogenation capacities of the catalysts. Moreover, the activation energy in the CH dehydrogenation is practically not modified by the Sn addition to Pt/ACF. Taking into account the structure-insensitive character of the CH dehydrogenation reaction (Cinneide and Clarke, 1972) it can be inferred that the very low modification of the activation energy as the Sn content increases would indicate either a negligible electronic change or a low alloy formation after tin addition to Pt. The cyclopentane hydrogenolysis is considered as a structure-sensitive reaction (Apesteguía and Barbier, 1982). In consequence, the low modification of the activity in the cyclopentane hydrogenolysis reaction (which displays a diminution about 40% for the PtSn (3 wt%)/ACF catalyst with respect to that of the monometallic one) can be interpreted as due to a low modification of the concentration of the Pt ensembles required for this reaction. This effect is different to that observed in PtSn/C catalysts with lower metal loadings (de Miguel et al., 2001).
From these results it can be concluded that the tin addition to Pt/ACF does not produce an important electronic modification of the metallic phase and does not modify in an important degree the hydrogenolytic capacity. This behaviour can be understood in terms of the intercalation of Sn atoms in the metallic surface but without an important change in the concentration of the hydrogenolytic sites.
Figure 2 shows the XPS of Sn 3d for PtSn/ ACF based catalysts reduced at 350o C. Besides, Table 4 compiles the XPS Pt4f7/2 and Sn 3d5/2 line positions and the percentage of the species with different oxidation states on samples pre-reduced "in situ" at 623 K under a H2 atmosphere. Besides the surface Sn/Pt atomic ratio obtained from the deconvolution of the corresponding spectra (see experimental section) is indicated in this table.
Table 4: XPS results of the different PtSn/ACF catalysts pre-reduced at 623 K corresponding to Pt 4f7/2 and Sn 3d5/2 levels. In each case, the peak position (BE) and the percentage of each species (between parenthesis), respectively, are indicated. Sn/Pt: surface Sn/Pt atomic ratio.

From the deconvolution of the XPS spectra of the different samples, it was obtained only one peak for the Pt 4f7/2 level at 71.2-71.6 eV. This peak can be assigned to zerovalent Pt (Wagner et al., 1993).
In the case of Sn 3d5/2, the deconvolution of the XPS spectra showed two peaks: one at 485.6-485.8 eV and another one at 486.8-487.3 eV. The first peak can be attributed to the presence of zerovalent Sn (Wagner et al., 1993). The second peak can be assigned to oxidized species of Sn (Sn II/IV). It must be indicated that, according to the literature, it is not possible to discriminate from XPS measurements between Sn(II) and Sn(IV), since both species show lines at the same BE (Wagner et al., 1993). Besides the literature (Wagner et al., 1993) gives higher binding energies values for SnCl2 (488 eV)than those reported in this paper. Hence, it is very difficult to consider the possible existence of this specie in our catalysts.
Table 4 also shows that the reducibility of Sn to Sn(0) appears to decrease when an important amount (3 wt%) of this component is added to Pt. Another aspect to be considered is the surface Sn/Pt atomic ratio (Sn/Pt in Table 4). In fact, the bulk Sn/Pt atomic ratiois: 0.33 for Sn= 1 wt%, 0.52 for 1.6 wt% and 0.99 for 3 wt%. By comparing these results with the corresponding surface Sn/Pt atomic ratio, it can be concluded that there is an enrichment of the metallic surface in Sn. However, this Sn enrichment is much lower than that found on PtSn/C catalysts with lower metallic loadings (0.30 wt% of Pt and 0.40 wt% of Sn). In fact, the surface Sn/Pt atomic ratio reported for the latter catalyst was higher than 20 (de Miguel et al., 2001). Hence, factors such as the nature of the support and the metallic loading can affect the structure of the metallic phase.
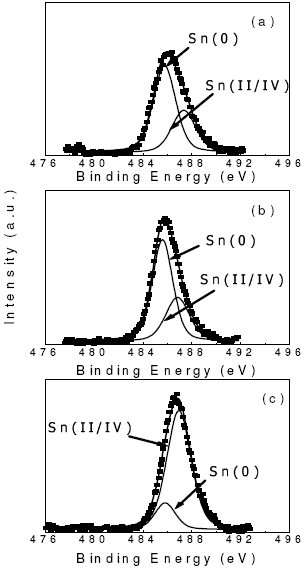
Fig. 2. Sn 3d5/2 XPS spectra of PtSn/ACF catalysts reduced at 350o C. (a) Pt(5 wt%)Sn(1 wt%)/ACF, (b) Pt(5 wt%)Sn(1.6 wt%)/ACF, (c) Pt(5 wt%)Sn(3 wt%)/ACF.
From these evidences, the model of the metallic phase of these PtSn/ACF catalysts appears to be complex. First, the negligible modification of the catalytic activity in citral hydrogenation, when increasing amounts of Sn are added to Pt, could be related to different opposite factors: 1) the presence of tin appears to enhance in an important degree the selective reduction of the carbonyl group of citral leading to a higher production of nerol and geraniol, 2) Sn addition would inhibit the hydrogenation of -C=C-bonds, which is reflected in a lower formation of citronellal, citronellol and the saturated alcohol (3,7 dimethyloctanol), 3) the production of isopulegol and acetals is also lowered when Sn is added to Pt, which can be assigned to a poisoning effect of Sn on the acidic sites of the support. The overall result of these effects is the low modification of the catalytic activity when the Sn content in the catalysts increases.
The selective reduction or hydrogenation of the carbonyl group and the inhibition of the hydrogenation of -C=C-bonds of citral would require a particular structure of the metallic surface (Homs et al., 2001). By assuming that ionic Sn species enhance the polarization of the carbonyl group, which then would react with the hydrogen dissociated on the Pt atoms, it can be inferred that ionic Sn species must be placed in the vicinity of the Pt atoms to produce this effect. In order to maintain the level of the hydrogenolytic activity when the Sn amount added to Pt is increased, the ionic Sn species would be intercalated between Pt atoms, thus producing new ensembles of Pt atoms. According to the results of the CP hydrogenolysis, the Sn addition to Pt/ACF produces a low modification of the hydrogenolytic capacity of the metallic phase. This means that the intercalation of Sn species between Pt atoms would lead to the formation of new ensembles. Taking into account the results showed in Table 5, it can be observed that H2 chemisorption values are slightly decreased when increasing amounts of Sn are added to Pt. This would mean that Sn is intercalated between Pt atoms. The size of these new ensembles after the Sn addition appears to be such as to inhibit the -C=C- hydrogenation, but it would be larger than that required for the hydrogenolysis reaction.
Table 5. H2 chemisorption capacity (H) of ACF-based catalysts.

Another topic related to the structure of the metallic phase is the formation of PtSn alloys. It has been reported in the literature (Aksoylu et al., 2000; Casella et al., 2000, de Miguel et al., 2001) evidences of the presence on these alloys. According to the results of CH dehydrogenation reaction, the activation energy values for PtSn/ACF catalysts are very close to that of the monometallic one, which indicate a low electronic effect between Pt and Sn, meaning a low concentration of PtSn alloy particles. XPS results show an important amount of metallic Sn after reduction. However, according to the results above mentioned, only a minor fraction of zerovalent Sn would be alloyed with Pt(0). The important concentration of Sn(0) detected in XPS measurements (mainly for catalysts with a Sn content up to 1.6 wt%) can be explained by assuming that Sn could be additionally reduced to Sn(0) by other effects such as H2 spill-over from Pt to Sn species placed in the interface between the support and the big metallic particles.
Moreover, XPS results show a certain enrichment of the surface by Sn. This effect together with the other evidences can be associated with an important amount of Sn (with different oxidation states) deposited on the support and another fraction of Sn intercalated between Pt atoms in the first layers of the big metallic particles.
IV. CONCLUSIONS
Tin addition to Pt/ACF strongly modifies the selectivity in the citral hydrogenation. In fact, Pt/ACF catalyst shows the formation of isopulegol, citronellal and acetals as the main products. The modification of the selectivity to nerol + geraniol by the tin addition to Pt can be associated to an important change in the structure of the metallic structure. These catalysts showed a better performance than Rh and Ir catalysts supported on silica (Singh and Vannice, 2001) and Rh catalysts supported on alumina (Mäki-Arvela et al., 2002).
On the basis of the test reaction results of the metallic phase, the catalytic behaviour in citral hydrogenation, H2 chemisorption experiments and XPS data, a model for the metallic surface can be supposed. In fact, the surface catalytic structure of PtSn/ACF catalysts can be described as having large metallic particles, where ionic Sn is intercalated between Pt atoms, but with a low blocking of Pt atoms by Sn on the particle surface. A low concentration of Pt alloys appears to exist on the metallic surface of the particles. Besides, an important fraction of tin would be deposited on the support with different oxidation states (Sn II/IV and Sn(0)). The surface Pt ensembles after tin addition to Pt/ACF would have the required size to maintain the activity in the cyclopentane hydrogenolysis in a similar level as that of the monometallic Pt/ACF catalysts. However, the Pt ensemble size appears to be smaller than the necessary to produce the hydrogenation of the double -C=C-bonds.
Finally, other alternatives can be explored in the future in order to obtain a more important modification of the selectivity to different products in the citral hydrogenation. Thus, the effect of the functionalization treatment by using other oxidant agents could lead to supports with different acid strength, and, in consequence, with a different capacity to produce compounds such as isopulegol.
Besides, different branched alcohols could be used in order to minimize the formation of acetals.
V. ACKNOWLEDGEMENTS
Authors wish to thank to Secretary of Science and Technology (Universidad Nacional del Litoral -CAI+D Program) and CONICET for the financial support, to M.A. Torres and E. Rincón, for the experimental help, and to Prof. F. Coloma by the experimental assistance in XPS measurements.
REFERENCES
1. Aksoylu, A. E., M. A. Freitas and J. L. Figueiredo, "Bimetallic Pt-Sn catalysts supported on activated carbon. I. Effect of the support modification and impregnation strategy", Appl. Catal. A: General, 192 (2000) 29-42.
2. Apesteguía, C.R., Barbier, J.,"Study on the hydrogenolysis of cyclopentane on supported metal catalysts. Effect of the sulphurization", Proceeding of the 8th. Iberoamerican Symposium on Catalysis, Spain, 1982, Vol II, p. 781.
3. Bachiller-Baeza, B., A. Guerrero-Ruíz, I. Rodríguez-Ramos, "Role of the residual chlorides in Pt and Ru catalysts for the hydrogenation of a,b unsaturated aldehydes", Appl. Catal. A 192 (2000) 289-297.
4. Baronetti, G. T., S. R.de Miguel, O. A. Scelza and A. A. Castro, "State of metallic phase in PtSn/Al2O3 catalysts prepared by different deposition techniques", Appl. Catal., 24 (1986), 109-116.
5. Casella, M. L., G. J. Siri, G. F. Santori and O. A. Ferretti, "Surface characterization of Li-modified Pt/Sn catalysts for isobutene dehydrogenation", Langmuir 13 (2000) 5639-5643.
6. Cinneide, A. D. and J. K. A. Clarke, "Catalysis on supported metals", Catal. Rev.; 7 (1972) 213-231.
7. de Miguel, S. R., M.C. Román-Martínez, D. Cazorla-Amorós, E. L. Jablonski and O. A. Scelza, "Effect of the support in Pt and PtSn catalysts used for selective hydrogenation of carvone", Catal. Today, 66 (2001) 289-295.
8. de Miguel, S.R., J. I. Vilella, E. L. Jablonski, O. A. Scelza, C. Salinas-Martínez de Lecea, A. Linares-Solano. Appl. Catal. A: Gen. 232 (2002) 237-246.
9. Didillon, B., J. P.Candy, F. Le Peletier, O. A. Ferretti and J. M. Basset, "Surface organometallic chemistry on metals. Selective hydrogenation of citral on supported Rhodium modified by tetra-n-butyl Germanium, Tin and Lead", in M. Guisnet et al. (Eds.), Heterogeneous Catalysis and Fine Chemicals III, Elsevier Sci. BV, (1993) 147-154.
10. Galvagno, S., C. Milone, A. Donato, G. Neri and R. Pietropaolo, "Influence of the metal particle size in the hydrogenation of citral over Ru/C", Catal. Lett., 18 (1993) 349-355.
11. Homs,N., J. Llorca, P. R.de la Piscina, F.Rodríguez-Reinoso, A. Sepúlveda Escribano and J. Silvestre-Albero, "Vapour phase hydrogenation of crotonaldehyde over Magnesia-supported Platinum –Tin catalysts", Phys. Chem. Chem. Phys. 3 (2001) 1782-1788.
12. S. Kogan, Landau, M. V., Herskowitz, M. And J. E. Koresh, "Shape -selectivity of Pt on carbon fibers catalysts", in M. Guisnet et al. (Eds.), Heterogeneous Catalysis and Fine Chemicals III, 1993, Elsevier Sci. Publishers B.V., p. 353-359.
13. Macías Pérez, M. C., C. Salinas Martínez de Lecea and A. Linares Solano, "Platinum supported on activated carbon cloths as catalyst for nitrobenzene hydrogenation", Appl. Catal. A: General, 151 (1997) 461-475.
14. Mäki-Arvela, P., L-P.Tiainen, A. Kalantar Neyestanaki, R. Sjöholm, K. Rantakylä, E.Laine, T. Salmi and D. Yu. Murzin, "Liquid phase hydrogenation of citral: suppression of side reactions", Appl. Catal. A: General 237 (2002) 181-200.
15. Malathi, R. and R. P. Visnawanath, "Citral hydrogenation on supported Platinum catalysts", Appl. Catal. A: General. 208 (2001) 323-327.
16. Neri, G., C. Milone, A. Donato, L. Mercadante and A. M. Visco, "Selective hydrog enation of citral over Pt-Sn supported on activated carbon", J. Chem. Technol. and Biotechnol. 60 (1994) 83-88.
17. Neri, G., C. Milone, S. Galvagno, A. P. J. Pypers and J. Schwank, "Characterization of Pt -Sn carbon hydrogenation catalysts", Appl. Catal. A: General, 227 (2002) 105-115.
18. Salmi, T., P. Mäki-Arvela, E. Toukoniitty, A. Neyestanaki, L-P. Tiainen, L-E. Linsfors, R. Sjöholm and E. Laine, "Liquid phase hydrogenation of citral over an immobile silica fibers", Appl. Catal. A: General, 196 (2000) 93-102.
19. Singh, U. K. and M. A. Vannice, "Liquid phase citral hydrogenation over SiO2-supported Group VIII-Metals", J. Catal., 199 (2001) 73-84.
20. Tiainen, L-P, Doctoral Thesis "Selective hydrogenation of citral on Nickel, Rhodium and Ruthenium Catalysts", Åbo Akademi, 1998.
21. Torres, G. C., E. L. Jablonski, G. T. Baronetti, A. A. Castro, S. R.de Miguel, O. A. Scelza, M. D. Blanco, M. A. P. Jiménez and J. L. G. Fierro, "Effect of the carbon pre-treatment on the properties and performance for nitrobenzene hydrogenation of Pt-C catalysts", Appl. Catal. A: General, 161 (1997) 213-226.
22. Vannice, M. A. and B. Sen, "Metal -support effects on the intramolecular selectivity of crotonaldehyde hydrogenation over Platinum", J. Catal., 115 (1989) 65-78.
23. Vilella, I. M. J., S. R de Miguel, C. Salinas Martínez de Lecea, A. Linares Solano and O. A. Scelza, "Bimetallic catalysts supported on different activated carbon materials for citral hydrogenation", Carbon 2003, p 318/313, 2003.
24. Wagner, C. D., Riggs, W. M., L.E. Davis and J. F. Moulder, "Handbook of X-ray photoelectronic spectroscopy" (1993). [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ]














