Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Latin American applied research
versão impressa ISSN 0327-0793
Lat. Am. appl. res. v.35 n.3 Bahía Blanca jul./set. 2005
Synthesis and characterization of poly (methyl methacrylate-styrene) copolymeric beads for bone cements
L. Morejón1, E. Mendizábal2, J. A. Delgado1, N. Davidenko1, F. López-Dellamary2, R. Manríquez2, M. P. Ginebra3, F. J. Gil3 and J. A. Planell3
1 Centro de Biomateriales, Universidad de La Habana. C. Habana, CP 10400, Cuba.
lizette@biomat.uh.cu
2 Universidad de Guadalajara, Jalisco. CP 44430. México
3 Universidad Politécnica de Cataluña, Barcelona, CP 08028. España.
Abstract — Acrylic bone cements have been used for about 40 years to fix artificial prosthesis to bone structure. The properties of the acrylic bone cement mostly depend on the characteristics of the beads, which are the main component of the solid part of the cement. In this work beads of poly(methyl methacrylate-co-styrene) were synthesized by suspension polymerization. The objective of this study was to obtain polymeric beads with particle size distributions and molecular weights that will permit the formulation of bone cements according to the international standards. Polyvinylpyrrolidone and polyvinylpyrrolidone-hydroxyapatite mixtures were studied as stabilizers of the system. Benzoyl peroxide (0.1, 1.0, 1.5% wt. in reference to the monomer) was the initiator of the reaction. The copolymeric beads were characterized by different analytical techniques. Polyvinylpyrrolidone alone was the best stabilizer and the bone cements prepared with polymer having low (247,000 g/mol) and high (800,000 g/mol) weight average molecular weights had static mechanical properties according to the requirements of commercial materials.
Keywords — Bone Cements. Poly(Methyl Methacrylate). Suspension Polymerization. Mechanical Properties.
I. INTRODUCTION
Acrylic bone cements are frequently used to fix the artificial prosthesis to the skeletal human system. The main application of bone cements is in the Total Hip Replacement. They have been used with this purpose for about 40 years (Charnley, 1964a, b). These materials main function are to secure of immediate anchorage of the implant and to permit a better distribution of body loads between the prostheses and the bone (Charnley, 1970; Planell et al., 1995).
Acrylic bone cements are composed of two parts, a liquid part: methyl methacrylate (MMA), N,N-dimethyl-p-toluidine (DMT) and hydroquinone (Hq) and a solid part composed by: acrylic beads, usually poly(methyl methacrylate) (PMMA) beads or their copolymers, benzoyl peroxide (BPO) to initiate the polymerization reaction and frequently they also include a radiopaque agent such as barium sulfate, or zirconium oxide.
The setting and mechanical properties of the acrylic bone cement depend on the characteristics of the beads, which are the main component of the solid part of the cement (Lee et al., 1973; Pascual et al., 1996). The polymeric beads constitute about 70% by weight of the polymerized cement. The diameter of most of the acrylic particles used for bone cement ranges between 30 and 150μm (Planell et al., 1995), however, there are reports where the average diameter varies between 10-30μm (Pascual et al., 1996). The shape of the particles is related to the manufacture process used (Planell et al., 1995). The molecular weight of the polymeric beads differs and depends on the cement commercial brand. The weight average molecular weight (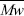 ) has values between 0.8x105 and 7x105 (Vila, 1992). It has been reported that some mechanical properties of the PMMA are independent of the molecular weight, for example abrasion and wear. Other properties such as tensile strength increases with the molecular weight up to 1.5x105 g/mol, above this molecular weight there is no change (Lautenschlager et al., 1987)
) has values between 0.8x105 and 7x105 (Vila, 1992). It has been reported that some mechanical properties of the PMMA are independent of the molecular weight, for example abrasion and wear. Other properties such as tensile strength increases with the molecular weight up to 1.5x105 g/mol, above this molecular weight there is no change (Lautenschlager et al., 1987)
Although there are some reports about the influence of the chemical composition, morphology, particle size distribution and molecular weight distribution in the final properties of the bone cement (Brauer, 1986, Zaldívar et al., 1991; Morejón, 1992, Pascual et al., 1996), the study of new stabilizers to obtain polymeric beads with optimal properties (morphology, particle size and distribution and polymer molecular weight and its distribution) is interesting because of its scientific and practical interest. In this work we analyzed the influence of the method to obtain the polymeric beads, on final properties of the bone cement. Copolymeric beads of poly(methyl methacrylate-co-styrene) P(MMA-ST) for bone cements were synthesized by suspension polymerization. In order to obtain adequate particle size distribution, two stabilizers of the suspension system were studied. We studied polyvinylpyrrolidone (PVP) as stabilizer of the suspension copolymerization of MMA and ST because by modifying its concentration and molecular weight it is possible to obtain beads with different particle size distribution (Morejón, 1992). On the other hand this stabilizer is easy to remove from the beads surface, which is very important for the medical applications of the product. The other stabilizer system analyzed was a polyvinylpyrrolidone-calcium phosphate mixture. The aim of studying this stabilizer was to introduce calcium phosphate particles in a homogeneous way in the conventional formulation of bone cements. It responds to the interest to obtain a final product with bioactivity, capable of stimulate bone reorganization in the surrounding of the implant and which will allow to obtain a secure and more durable anchorage of the prosthesis. On the other hand, there are reports that mention that inorganic salts, for example, calcium phosphate powders, can provide an appropriate and clean stabilization of the monomer drops in the suspension polymerization for medical and industrial applications (Bishop, 1971). In this work the experiments were carried out with polyvinylpyrrolidone-hydroxyapatite (PVP-HA) suspensions. The HA was synthesized in situ previous to the polymerization by the reaction between Ca(OH)2 and H3PO4.
Surgery materials should be sterilized and the sterilization process might change the molecular weight of the polymer and therefore its properties. For example, the Ethylene Oxide sterilization process does not affect the molecular weight of the polymeric beads but leaves residuals; on the other hand, Gamma Irradiation affects the molecular weight of the beads, but it is a clean technique. Then, it is very necessary to be able to obtain polymer beads with a wide spectrum of molecular weights in order to select the optimal composition depending on the sterilization method. For this reason another aim of this work was to obtain bone cements containing polymer with different molecular weight distributions and for this reason the influence of the initiator concentration on molecular weights was examined. The possibility for obtaining polymer with different molecular weight distributions will increases the spectrum of applications of these materials for different clinical cases
The P(MMA-ST) copolymeric beads synthesized were characterized by Optical Microscopy (OM), Gel Permeation Chromatography (GPC), Differential Scanning Calorimetry (DSC) and Nuclear Magnetic Resonance (NMR-1H, NMR-13C). Bone cements were prepared using a conventional composition and employing a 2/1 solid/liquid volume ratio. Setting times and static mechanical properties (compression strength, tensile strength and fracture toughness) of the bone cement formulations were determined.
II. MATERIALS AND METHODS
suspension polymerization was used to synthesize the acrylic beads. This technique consists on dispersing a monomer as droplets in a continuous phase and polymerizing in that way (Fig. 1). Usually an aqueous continuous phase is used and the monomer (s) is water insoluble. In our case the monomers are methyl methacrylate and styrene. We also used a suspension stabilizer and a vigorous stirring to maintain the stability of the system, that means avoid coalescence and agglomeration of the beads during the polymerization reaction. The ratio of methyl methacrylate/styrene monomers was 80ml/20ml. Stirring speed was 700 r.p.m. and reaction time was 24 h. The ratio of organic/aqueous phase was 1/3. Depending on the benzoyl peroxide concentration used (0.1, 1.0, 1.5 % of BPO) different temperature-time steps were used (Table 1). These temperature increment steps were used to prevent bead agglomeration and to obtain a high degree of conversion in order to minimize residuals of monomer and BPO. Table 2, shows the polyvinylpyrrolidone (PVP-K-90) and PVP-HA mixtures used as stabilizers.

Figure 1. Schematic representation of suspension polymerization
Table 1. Temperature-time steps depending on the BPO concentration used
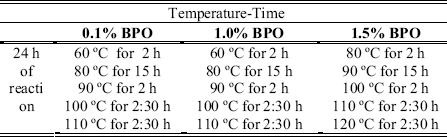
Table 2. Polyvinylpirrolidone-hydroxyapatite mixtures
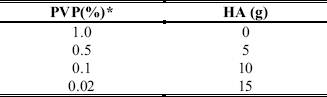
*Weight percent in reference to the aqueous phase
Optical Microscopy and Light Scattering were used to analyze the particle size distribution of beads. The molecular weight distribution was determined by Gel Permeation Chromatography (Perkin Elmer Chromatographer). The beads were examined by Differential Scanning Calorimetry (DSC) in order to determine the Tg values. The heating speed was 20 oC/min. The composition of the copolymer obtained was determined using NMR-1H and NMR-13C with a Varian Equipment Geminis 2000 at 200MHz in deuterated chloroform (5 %w/v) as solvent and tetramethylsilane (TMS) as internal standard.
Bone cements formulations
The bone cement solid part was prepared with 88.0% (weight percent) of copolymeric P(MMA-ST) beads, 2.0% of benzoyl peroxide and 10.0% of barium sulfate. The liquid part had 99.0% (weight percent) of methyl methacrylate, 1.0% of N,N dimethyl-p-toluidine and 80 ppm of hydroquinone. In all cases an 2/1 solid/liquid ratio was used.
Setting properties and Mechanical Assays
To determine setting time (St) and the temperature peak (maximum polymerization temperature, Tmax) the materials were mixed and put into an ultra high molecular weight polyethylene cylindrical mould, which was designed to obtain reproducible data using a minimum amount of cement mixture. The temperature was measured every 10 seconds with an Ω Omega® DP465 Silver Plated thermocouple inserted in the center of the mass volume. The setting time was calculated as the time when the temperature reaches the value:  , being Tenv the enviromental temperature.
, being Tenv the enviromental temperature.
The compression strength of the cement was determined according to the specifications of ISO-5833 standard (ISO 5833, 2002). The specimens dimensions were 12 mm and 6 mm in height and diameter and the tests were carried out at a constant cross-head speed of 20mm/min. The tensile strength experiments were carried out using halterium plane probes elaborated according to the ASTM-638 standard (ASTM D 638, 1991). The dimensions of the test specimens are shown in Fig. 2. The cross-head speed employed was 1 mm/min. Compression and tensile testing were carried out in a United 5802 Universal Testing Machine. Each sample was measured and stored for 24 ± 2 h in dry conditions at 23 ± 2 oC prior testing. At least five specimens were tested, and the mean and standard deviation is reported for each sample.
Fracture toughness was measured using compact tension (CT) test specimens prepared following ASTM 5045 standard (ASTM D5045, 1999). The cement formulations were prepared at room temperature and were manually inserted into a teflon mould at approximately dough time. The mould was closed and the mixture was kept there for one hour at 37 ± 0.1 oC. The blocks obtained were subsequently machined to the specified dimensions (Fig. 2). The notch tip was sharpened with a razor blade. The tests were performed after a month of sample preparation using a Universal Testing Machine BIONIX 858 Test System MTS with an elongation speed of 1 mm/min. The fracture toughness, K1c, corresponding to elastic-plastic fracture mechanics conditions is given by:
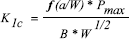 | , (1) |
where Pmax is the peak load during the test, B is the thickness of the specimen and f(a/w) is a geometrical factor. In all cases it was verified the fulfillment of conditions of plane deformation in the crack tip (Hertzberg,1996):
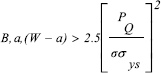 | , (2) |
where σys is the elastic limit stress and PQ is found by the intersection of the stress-strain curve with a secant line having a slope 5% lower than that of the linear region. f(a/w) was calculated by:
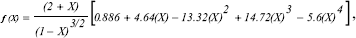
where X=a/W.

Figure 2. Tensile and Fracture Toughness specimens dimensions.
III. RESULTS AND DISCUSSION
Particle Size Distribution
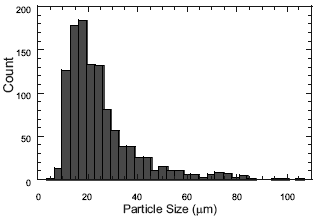
Morphologic analyses of the beads showed that the particles were spherical for both stabilizers. When using PVP as stabilizer, beads with clean surfaces, unimodal particle size distribution and average diameters between 24 and 34 μm (which are on the range of particle size of commercial materials) were obtained (Fig. 3a.)
When using PVP-HA as stabilizer particle size distribution was bimodal and the average size of the beads was higher than 100 μm fact that makes difficult the handle of the bone cements (Fig. 3b). Larger size was obtained because the amount of PVP was reduced and the HA was not as effective as the PVP as stabilizer. The surface of beads had inlays of HA. Optical Microscopy indicated that the higher concentration of HA as stabilizer, the higher amount of HA particles adhered to the beads surfaces. This is shown in Fig. 4, where the beads were completely covered with HA in a 0.02% PVP-15g HA system as stabilizer. The fact that the polymeric particle size was excessively increased when using the PVP-HA stabilizer in all formulations, limits the use of calcium phosphate as a suspension stabilizer of the beads for bone cements. For this reason we only increased the concentration of HA in the PVPHA mixtures as stabilizer until 15g (Table 2).
a)
b)
 s
s
Figure 3. Particle size distribution of the copolymeric beads. a) 1.0% PVP (P3). b) 0.1%PVP-10gHA (P6).

Figure 4. Optical Microscopy of a P(MMA-ST) bead obtained with 0.02% PVP-15g HA.
Table 3. Number average diameter and Size Range of the P(MMA-ST) beads obtained with different initiator concentration (BPO) and different stabilizer system.

Table 3 presents the number average diameter of beads obtained with 1.0% PVP and 0.1% PVP-10.0g HA mixtures at different concentration of initiator (BPO). It is observed a slight increment in the average diameter with the increment of initiator concentration but there were no statistical differences. The beads obtained when using HA as stabilizer were too big for to be used as part of the formulation of bone cements.
Molecular Weight Distribution
As expected, the molecular weight of the polymer decreases with the increase of initiator concentration due to the higher concentration of free radicals in the system. Under the studied conditions, average weight molecular weights (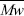 ) between 232 000 and 850 000 g/mol (Table 4) were obtained. In all cases molecular weight distributions were unimodal and polydispersity values between 1.6-3.4 were obtained.
) between 232 000 and 850 000 g/mol (Table 4) were obtained. In all cases molecular weight distributions were unimodal and polydispersity values between 1.6-3.4 were obtained.
Table 4. Average Molecular Weight (g/mol) of P(MMA-ST) beads according to initiator concentration and stabilizer type for two batch of each system

Figure 5 shows the differential molecular weight distributions as a function of the initiator concentration when using 0.1%PVP-10.0gHA experiments. It can be seen that the distributions are unimodal and that molecular weight increases with the decrease of the initiator concentration. By comparison the average molecular weight for the solid part of the commercial bone cement Surgical Simplex P was determined:  = 9.98 *104 g/mol,
= 9.98 *104 g/mol, 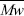 = 2.32 *105 g/mol,
= 2.32 *105 g/mol,  = 3.63 *105 g/mol.
= 3.63 *105 g/mol.
Taking into account the obtained results, the subsequent studies (characterization studies, preparation of bone cement formulations, and mechanical assays) were carried out with P1 and P3 samples which were samples with adequate size particle distributions and extreme (the highest and the lowest) values of average molecular weight. It was verified that the cement formulations produced homogeneous dough and were easily handled. The manipulability characteristics of each cement composition have a special importance, because they are a qualitative parameter that allows identifying whether those compositions can be used for practical formulations.

Figure 5. Differential molecular weight distribution as a function of initiator concentration when using 0.1%PVP-10.0g HA stabilizer
Spectroscopic Characterization of the P(MMA-ST) beads.
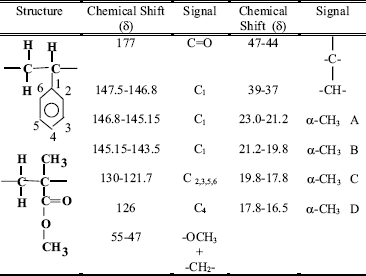
The synthesized P(MMA-ST) beads were characterized by NMR-1H, and NMR-13C. Figure 6 shows a typical NMR-1H spectrum of the copolymeric beads which corresponds to the statistical distribution of the monomeric units along the copolymer chains (Aerdts et al., 1993). The copolymer composition was determined by comparing integrated intensities of resonance signals and chemical shifts, Table 5.

Figure 6. NMR-1H spectrum of P3 beads
The monomeric distribution in the polymeric chain depends on the reactivity ratios r1=k11/k12 y r2=k22/k21, where k11 and k12 are the specific rate constants of homopolymerization and k12 and k21 are the specific rate constants of copolymerization. In the radicalic copolymerization of MMA and ST the reactivity ratios are very similar: 0.46 and 0.52 at 60oC, and 0.59 and 0.54 at 131oC respectively (Odian, 1991). The reactivity ratios are inferior to the unit, which indicates that each monomer has a natural tendency to react with the other monomer. However, for the reaction studied there is a larger amount of methyl methacrylate monomer in the feed mixture (80MMA/20ST) and the reaction is not carried out in conditions that allow a strict alternation of the monomers. For this reason the polymer obtained was statistical.
Table 5. NMR-1H chemicals shifts and signal assignations of P3 beads.

The fact that the polymers have a statistical distribution of the monomers along the chain means that an important spatial regularity is not probable. If a polymer is perfectly alternated MMA-ST, it can be stated that they have three types of space isomerism for each triad: MMA-ST-MMA, or, ST-MMA-ST (Fig. 7).

Figure 7. Space isomerism of ST-MMA-ST triad.
However, for a statistical polymer 20 space configurations existed, 10 for the triads centered in styrene and 10 for the triads centered in methacrylate with different configurational sequences, for example: centered triads in MMA:
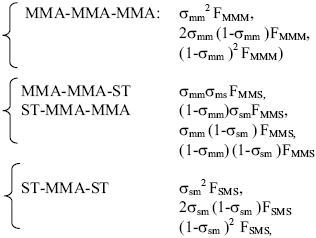
where: F is the molar fraction for each type of triad and σ is named coisotacticity parameter and it is the probability that two monomeric units have isotactic configuration. It is reported (Aerdts et al., 1993) that V has the following values: σmm=0.23, σss=0.29, σsm=0.44.

Figure 8. NMR-13C spectrum of P3 beads.
The NMR-13C spectra showed tree types of stereoisomers (Fig.8). The α-CH3 and C1 groups indicated the presence of these three different relative positions in the space of the groups. In the case of α-CH3 groups four picks denominated A,B,C,D: A, 23.0-21.2 ppm; B, 21.2-19.8 ppm; C, 19.8-17.8 ppm; D, 17.8-16.5 ppm can be seen. In this case, the assignation of the signal is not clear. However, the C1 signal is divided in three picks, the assignation in this case is already reported (Maxwell et al., 1993): X, 147.5-146.8ppm Sindyotactic; Y, 146.8-145.15ppm Atactic and Z 145.15-143.5ppm Isotactic (Fig. 9). In this way it can be concluded that in the synthesized polymer there are three space configurations: Syndiotactic, Atactic and Isotatic. Table 6 summarizes the NMR-13C chemicals shifts and signal assignations.
The glass transition temperature (Tg) determined for the P1 and P3 polymer samples were 112.5 ± 1.2oC and 112.9±0.8oC respectively, which confirms that the spatial structure is due to a space isomers mixture. Other authors reported (Encyclopedia of Polymer Science and Engineering, 1985) that the Tg values were 105 oC for Atactic, 160 oC for Syndiotactic and 43 oC for Isotactic copolymers. For bone cement materials 52.6% of Syndiotactic, 37.42% of Atactic and 3.6% of Isotactic isomers have been described (Stupp and Yau, 1981).
Another conclusion that is derived from the Tg values is that there are not significant differences between the P1 and P3 structures although the average molecular weight 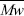 of P1 is three times higher than the average molecular weight
of P1 is three times higher than the average molecular weight 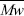 of P3. Some reports indicate that certain polymeric properties become independent of the molecular weight starting from certain values (Lautenschlager et al., 1987).
of P3. Some reports indicate that certain polymeric properties become independent of the molecular weight starting from certain values (Lautenschlager et al., 1987).
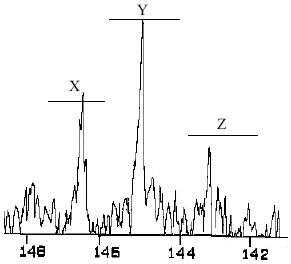
Figure 9. C1 signal amplified zone of the NMR-13C spectrum of P3 beads
Table 6. NMR-13C chemicals shifts and signal assignations
Setting Time and Temperature Peak.
Curing of the acrylic cement is a complex process because the contact between the liquid and the solid part marks the onset of several physical and chemical events that take place simultaneously. The solvation of the polymeric beads by the monomer, dissolution of the free radical initiator, diffusion from the liquid to the solid phase and monomer evaporation from the mixture are some of the physical events that occurs (Lautenschlager et al., 1987). The most important chemical process is the free radical polymerization of the MMA. Furthermore, due to the presence of hydroquinone and oxygen (inhibitors), which capture the free radicals produced by the initiator, there is always present an induction time. After inhibitors are consumed, polymerization reaction starts, the monomer is rapidly consumed by the propagation rate and the viscosity increases, producing an increment in reaction rate and in temperature because of the so called "gel effect" (Odian, 1991). The viscosity of the curing mixture increases rapidly because of the dissolution of the beads of PMMA (the smaller the beads, the larger the ratio of surface area/volume, the faster their dissolution and the larger the molecular weight the larger the increase in viscosity) and by the conversion of the monomeric units into large polymeric chains. Therefore, the setting time of the curing of acrylic cements depends on diverse factors, among them: the initiator and inhibitors concentration of the formulation, the properties of the polymeric beads (particle size and molecular weight distribution) that determined the proportion of polymer dissolved in the monomer and of course the humidity and the environmental temperature. According to the ISO and ASTM international standards the setting time of the acrylic bone cement should be between 3-15 min and 5-15min respectively. The polymerization of acrylic bone cement is an exothermic reaction and the calculated heat of polymerization of MMA is 544J/g (Meyer et al., 1973). The thermal effects of the polymerization are reflected in a significant temperature peak (ranging between 80oC and 124oC in the cement (Saha and Pal, 1984) and between 48oC and 105oC at the bone/cement interface (Brauer et al., 1986). It has been reported that the polymerization temperature of acrylic bone cement can cause necrotic areas in the surrounding of the prosthesis (Meyer et al., 1973). The thermal damage in the bone can affects the durability of the implant, because it induces bone reabsortion and produces aseptic loosening of the prosthesis. In order to minimize the thermal necrosis of the adjacent tissues and to promote a long-time good fixation the international standards established that temperature peak of bone cement curing should not overcome the 90oC. The values of the setting time and the temperature peak obtained from the experiments are shown in Table 7. It can be seen that temperature peaks are low, which is important to prevent tissue damage. Similar setting times were obtained for both formulations. However, since that particle size is smaller (and molecular weight is larger) for P1 than for P2, then the medium viscosity will be higher. This results in a bigger "gel effect" and a higher temperature peak. Both samples fulfill the requirements of setting time and the temperature peaks of polymerization are low.
Mechanical Testing
Adequate mechanical properties of bone cements are essential to avoid the failure of the hip prosthesis during their use. There are numerous reports in the scientific literature about these properties. For the commercial cements they report the following values: Compression strength: 77 to 103 MPa (Freitag and Cannon, 1976; Hansen and Steen, 1992; Topoleski et al., 1993; Kühn, 2000), Tensile strength: 24.5-64.9 MPa (Lee and Ling, 1975; Pilliar et al.,1976; Pourdeyhimi and Wagner, 1989) and Fracture Toughness: 0.87 to 1.59 MPa m1/2 (Freitag and Cannon, 1976; Starck, 1979; Sih and Berman, 1980; Vila et al., 1990). In Table 7 mechanical properties of the cements are presented. In all cases the cement samples overcome the minimum requirements of the standards and they are in agreement with the values reported for commercial materials. The results obtained here show that in the range of molecular weights studied 230 000-850 000 g/mol the mechanical properties are independent of molecular weight of the polymer in the beads
This work demonstrates that it is possible to obtain a wide range of molecular weights for the P(MMA-ST) beads that satisfy the required mechanical properties. This method is effective to prepare P(MMA-ST) beads for different medical applications. Also, this technique allows obtaining beads containing polymer with an appropriate molecular weight for a specific sterilization method.
Table 7. Properties of experimental bone cements

IV. CONCLUSIONS
It was demonstrated that the two stabilizers studied (PVP and PVP-HA mixtures) are effective to avoid the coagulation during the suspension polymerization process to obtain P(MMA-ST) beads. However, though the morphological analysis revealed spherical particles when using both stabilizers, only PVP provides the beads with unimodal distribution in the range of sizes of commercial cements: 10-30 μm. In the case of the PVPHA stabilizer the distribution obtained is bimodal and the average bead size is higher than 100 μm. This excessive bead size prevents the use of this stabilizer in the synthesis of beads for acrylic bone cements. With initiator concentrations between 0.1-1.5% and under the reaction conditions used, polymers with weight average molecular weights ( ) in a range between 230 000 and 850 000 g/mol were obtained, which is very useful for the preparation of cements for different medical applications. In all cases the molecular weight distributions were broad, symmetrical and unimodal. The polydispersity values were between 1.6 and 3.4. The experimental bone cements have setting and mechanical properties in agreement with the requirements of international standards.
) in a range between 230 000 and 850 000 g/mol were obtained, which is very useful for the preparation of cements for different medical applications. In all cases the molecular weight distributions were broad, symmetrical and unimodal. The polydispersity values were between 1.6 and 3.4. The experimental bone cements have setting and mechanical properties in agreement with the requirements of international standards.
V. REFERENCES
1. Aerdts, A.M., J.W. De Haan and A.L. German, "Proton and Carbon NMR of Alternating and Statistical Sturene-Methyl Methacrylate Copolymers Revisited", Macromolecules, 26, 1965-1971 (1993). [ Links ]
2. ASTM D 638-1991. Standard Method for Tensile Properties of Plastic (1991). [ Links ]
3. ASTM D5045-99 Standard Test Methods for Plane-Strain Fracture Toughness and Strain Energy Release Rate of Plastics Materials (1999). [ Links ]
4. Bishop, R.B., Practical Polymerization for Styrene, Cahners Books, Massachusetts (1971). [ Links ]
5. Brauer, G.M., D.R. Steinberger and J.W. Stansbury, "Dependence of curing time, peak temperature, and mechanical properties on the composition of bone cement", J. Biomed. Mater. Res., 20, 839-852 (1986). [ Links ]
6. Charnley, J., Acrylic cement in Orthopaedic Surgery. Edinburgh-London: E. and S. Livingstone. Williams and Wilkins Editors, Baltimore (1970). [ Links ]
7. Charnley, J., "Anchorange of femoral head prosthesis to shaft of the femur", J. Bone. Joint Surg., 42B, 28-30 (1964a). [ Links ]
8. Charnley, J., "Bonding of Prostheses to bone by cement", J. Bone. Joint Surg., 46B, 518-528 (1964b). [ Links ]
9. Encyclopedia of Polymer Science and Engineering: Second Edition, Vol 1, John Wiley & Son, New York, 256 (1985). [ Links ]
10. Freitag, T.A. and S.L. Cannon, "Fracture characteristics of acrylic bone cements. I. Fracture toughness", J Biomed Mater Res, 10(5), 805-828 (1976). [ Links ]
11. Hansen, D. and J. Steen, "Additional mechanical tests of bone cements", Acta Orthop Belg,; 58(3), 268-271, (1992). [ Links ]
12. Hertzberg, R.W., Deformation and Fracture Mechanics of Engineering Materials, 4th Ed., John Wiley & Sons, New York, (1996). [ Links ]
13. ISO 5833-2002. International Standard, Implants for Surgery-Acrylic resin cements (2002). [ Links ]
14. Kühn, K. Bone cements: Up- to date comparison of physical and chemicals properties of commercial materials, Springer-verlag Berlin Heidelberg, (2000). [ Links ]
15. Lautenschlager, E.P., S.I. Stupp and J.C. Keller, "Structure and Properties of Acrylic Bone Cement", in Functional Behaviour of Orthopaedic Biomaterials, Eds. P. Ducheyne y G.W. Hastings, CRC Press Boca Raton, Florida, 87-117 (1987). [ Links ]
16. Lee, A.J.C., R.S.M. Ling and J.D. Wrightson, "Some properties of poly(methyl methacrylate) with reference to its use in orthopaedic surgery", Clin. Orthop., 95, 281-287 (1973). [ Links ]
17. Lee, A.J.C. and R.S.M. Ling, "Further studies of monomer loss by evaporation during the preparation of acrylic cement for use in orthopaedic surgery", Clin. Orthop. Relat. Res., 106, 122-125 (1975). [ Links ]
18. Maxwell, I.A., A.M. Aerdts and A.L German, "Free Radical Copolymerization: An NMR Investigation of Current Kinetic Models", Macromolecules, 26, 1956-1964 (1993). [ Links ]
19. Meyer, P.R., E.P. Lautenschlager and B.K. Moore, "On the Setting Properties of Acrylic Bone Cement", J. of Bone and Joint Surgery, 55-A (1), 149-156 (1973). [ Links ]
20. Morejón, L. "Obtaining and Characterization of new versions of Surgical Cements", Thesis of University Degree, University of Havana, Havana, Cuba (1992). [ Links ]
21. Odian, G., Principles of Polimerization, Cap 6, 3rd Edition, John Wiley & Sons, Inc, New York (1991). [ Links ]
22. Pascual, B., B. Vázquez, M. Gurruchaga, M. Goñi, M.P. Ginebra, F.J. Gil, J.A. Planell, B. Levenfeld, and J. San Roman, "New aspects of the effect of size and size distribution on the setting parameters and mechanical properties of acrylic bone cements", Biomaterials 17(5), 509-516 (1996). [ Links ]
23. Pilliar, R.M., R. Blackwell, I. Macnab and H.U. Cameron, "Carbon-fiber reinforced bone cement in orthopaedic surgery", J. Biomed. Mater. Res., 10, 893-906 (1976). [ Links ]
24. Planell, J.A., M.M. Vila, F.J. Gil and F.C.M. Driessens, "Acrylic Bone Cements", Wise DL, Trantolo DJ, Altobelli DE, Taszewski MJ, Gresser JD, Schwantz ER, editors. Encyclopedic Handbook of Biomaterials and Bioengineering, Part B: Applications, Publisher Marcel Dekker Inc., New York, 879-921 (1995). [ Links ]
25. Pourdeyhimi, B. and H.D. Wagner, "Elastic and ultimate properties of acrylic bone cement reinforced with ultra high molecular weight polyethylene fibres", J. Biomed. Mater. Res., 23, 63-80 (1989). [ Links ]
26. Saha, S. and S. Pal, "Mechanical properties of bone cement: A review.", J. Biomed. Mater. Res., 18, 435-462 (1984). [ Links ]
27. Sih, G.C. and A.T. Berman, "Fracture toughness concept applied to methyl methacrylate", J. Biomed. Mater. Res., 14(3), 311-324 (1980). [ Links ]
28. Starck, C.F. "Fracture and fatigue characteristics of acrylic bone cement", J. Biomed. Mater. Res.; 13, 339-342 (1979). [ Links ]
29. Stupp, S.I. and H.L. Yau, "Matrix Polymerization and Stereoregularity of Poly(methacrylates)", University of Illinois Polymer Symposium, Urbana, III (1981). [ Links ]
30. Topoleski, L.D.T., P. Ducheyne and J.M. Cuckler, "Microstructural pathway of fracture in poly(methyl methacrylate) bone cement", Biomaterials, 14 (15), 1165-1172 (1993). [ Links ]
31. Vila, M.M., Ph.D. Thesis, Optimización de la Fijación de Prótesis-Hueso en implantes articulares cementados, Universitat Politècnica de Catalunya , Spain (1992). [ Links ]
32. Vila, M.M., A. Raya and J.A. Planell, "Mechanical behaviour of a rubber modified bone cement", Advances in Biomaterials, 9, 155-160 (1990). [ Links ]
33. Zaldívar, D., M.E. Cohen, C. Peniche, P. Ortiz and G. Müller, "Influence of molecular weight on the embedding capacity of poly(methyl methacrylate) and poly(methyl methacrylate- styrene) copolymer", Revista CENIC Ciencias Químicas 22 (2-3), 5-7 (1991). [ Links ]














