Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Latin American applied research
Print version ISSN 0327-0793
Lat. Am. appl. res. vol.35 no.3 Bahía Blanca July/Sept. 2005
In vitro model to study arterial wall dynamics through pressure-diameter relationship analysis
D. Bia1, R. L. Armentano1,2, Y. Zócalo1, W. Barmak2, E. Migliaro1, E. I. Cabrera Fischer2,3
1 Physiology Department, School of Medicine, Universidad de la República, General Flores 2125, Montevideo, Uruguay, CP:11800.
dbia@fmed.edu.uy
2 Favaloro University, Solís 453, C1078AAI, Buenos Aires, Argentina.
fischer@favaloro.edu.ar
3 Member of the Research Career, CONICET, Buenos Aires, Argentina.
Abstract — This work describes the biophysical basis of blood vessels' wall dynamics and reports a methodology developed in our laboratory to characterize mechanical vessels' wall properties and those of vascular prostheses. Our study includes in vitro measurements of arteries, veins and ePTFE conduits placed in a circulating loop. Segments are allowed to equilibrate for a period of 15 minutes under a steady state of flow (150 ml/min) and a mean pressure of 93 mmHg, at a stretching rate of 110 beats/min.
Data analysis consisted in obtaining pressure-diameter loop in order to calculate: Incremental elastic modulus, wall viscosity, Peterson modulus, pulse wave velocity, characteristic impedance, stiffness index, cross sectional compliance and distensibility.
Incremental elastic modulus of ePTFE (48.56±0.82 107dyn/cm2) was significantly higher than that of the veins (26.19±19.90 107dyn/cm2) and that of the arteries (4.06±2.55 107dyn/cm2).
This is an important approach, since mechanical wall dynamics plays a major role in vascular disease.
Keywords — Arterial Wall. Circulating Loop. Viscosity. Elasticity.
I. INTRODUCTION
The main function of the systemic circulation is to hold a constant blood flow through the capillary vessels. One of the determinants of left ventricular performance is the mechanical behavior of systemic arteries. In those vessels with large diameters, the mechanical properties are mainly set by the viscoelastic individual contribution of each structural constituent (Armentano et al., 1995a).
It has been reported that arterial diseases, such as human hypertension and atherosclerosis, are associated with modifications of physical properties of large arteries (Armentano et al., 1998). Besides, biological or synthetic tubular segments used as vascular prostheses are characterized by a high elastic modulus and cause modifications in the arterial wall-blood dynamics. In fact, viscoelastic properties of saphenous vein or expanded polytetrafluoroethylene (ePTFE) conduits usually used for coronary and peripheral vascular bypass grafting, differ from the native artery resulting in a mechanic mismatch that determines the development of intimal hyperplasia (Armentano et al., 2004).
The aim of this work is to describe the biophysical basis of blood vessels' wall dynamics and to report the methodology developed in our laboratory to characterize mechanical vessels' wall properties and those of vascular prostheses. Experimental results will also be provided.
II. STRUCTURAL INTEGRATION OF THE ARTERIAL WALL
The wall of the arteries consists of a tunica intima, tunica media and tunica adventitia. The intima is the innermost layer and is composed of a single layer of squamous endothelial cells, a thin basal lamina and a subendothelial layer composed by collagen, smooth muscle cells and fibroblasts (Clark and Glagov, 1985).
The tunica media is composed of smooth muscle cells, elastic sheets and collagenous fibrils. In the human being the number of elastic lamellae is related to the anatomic location of the artery; muscular arteries have only one internal and external elastic lamina while in the aorta there are about 60-90 elastic laminae. Their number decreases gradually toward the periphery of the arterial segment (Wolinsky and Glagov, 1967).
Figure 1 shows that, arterial elastic lamellae and smooth muscle cells are wrapped by a network of collagenous fibrils. An elastic lamina is concentrically arranged such that collagen bundles are better recognized though electron micrograph. Most of the collagen fascicles are oriented circumferentially but some are oriented obliquely and others longitudinally (Clark and Glagov, 1985). This lamellar unit of arterial medial structure contributes to the mechanical properties of the arterial wall.
The tunica adventitia is the outermost layer of the arterial wall and consists of dense fibroelastic tissue, vasa vasorum and nerves. This tunica is very important because the vasa vasorum supplies most of tunica media and adventitia with nutrients. The external elastic lamina is the limit between the tunica media and adventitia layer (Wolinsky and Glagov, 1969).

Figure 1. Schematic representation of the medial layer of the arterial wall. In arteries with only one elastic lamina, the latter serves as the border of the intima layer (in contact with the blood flow). The elastic lamina and smooth muscle cells are held together by a network of collagenous fibrils.
III. ARTERIAL WALL MECHANICAL BEHAVIOR
Arterial wall dynamic properties depend on the mechanical role exerted by passive components (elastin and collagen fibers) and active components (vascular smooth muscle cells). These components determine the elastic, viscous and inertial properties of the vessel, being the inertial component negligible (Armentano et al., 1995b; Wolinsky, 1970).
Mechanical properties of the arterial wall can be quantitatively analyzed using instantaneous pressure-diameter recordings, both in experimental preparations and in clinical non-invasive studies, as previously reported (Armentano et al., 1995a; Armentano et al., 1998; Bia et al., 2003; Bia et al., 2004; Cabrera Fischer et al., 2002). To the best of our knowledge, arterial pressure-diameter or stress-strain instantaneous loops determination is the most appropriate technique to assess the dynamic behavior of arterial wall constituents (Cabrera Fischer et al., 1991; Armentano et al., 1995a, b).
The most important mechanical property of the artery wall is its non-linear elasticity. Such vascular elasticity is a key determinant of blood flow dynamics in any circulatory system. Elastin and collagen fibers contribute individually to the whole arterial elasticity. And the elastic modulus of the arterial wall is composed of the elastic modulus of elastin fibers, the elastic modulus of collagen fibers and the recruitment of collagen fibers supporting the wall stress (Barra et al., 1993).
Viscosity is the force required to overcome a lack of slipperiness and it has been called "internal friction" (Folkow and Neil, 1971). All components of the arterial wall may contribute to its viscosity, but the smooth muscle is the only to be able to respond to a physiological stimulus. An increased arterial wall viscosity has already been reported in vessels corresponding to both hypertensive animals and patients (Dobrin, 1978; Barra et al., 1997; Armentano et al., 1998). Therefore, characterization of the viscous behavior of the arterial wall could widen the knowledge of pathological factors that determine the genesis of atherosclerotic plaques. The clinical implication of the non-invasive measurements of arterial wall viscosity could be highly relevant to identify high risk populations.
Several models have been used in order to characterize the viscoelastic properties of the arterial wall (Barra et al., 1993; Bia et al., 2004; Armentano et al., 1995a). An important determinant of arterial wall mechanical behavior is its viscosity which involves smooth muscle activity (Bia et al., 2004). An understanding definition of arterial wall viscosity is that which considers it as the lag of pulsatile diameter change behind applied pressure (O'Rourke, 1982). Therefore, it can be represented as the hysteresis loop when pressure and diameter are plotted against each other (O'Rourke, 1982; Armentano et al., 1998; Weterer et al., 1978; Bauer et al., 1979; Armentano et al., 1995a). See Fig. 2.
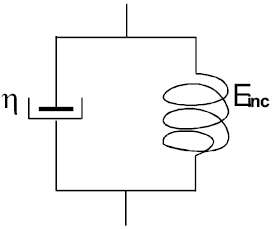
Figure 2. Viscous (η) and elastic (Einc) properties of the arterial wall are considered in this model (Kelvin-Voigt model). Low mass of arteries, veins and vascular prostheses determine a negligible value of inertial modulus.
Arterial wall viscosity has been reported to play a major role in regulating the mechanical behavior of muscular arteries both in animal preparations and humans (Gow and Taylor, 1968; Imura et al., 1990). The major effect of arterial wall viscosity on arterial mechanical properties is the alteration of the arterial pulse wave. We are concerned with the way in which it has been described in literature: as an attenuation similar to that caused by blood within the vessel, or as a dampening higher to that due to the viscosity of the blood alone (Nichols and O'Rourke, 1998), or as an element with a minor role in the vessel wall mechanics (Giezeman et al., 1994).
As we have pointed out above, the location of viscous elements in the arterial wall is largely represented by the smooth muscle component (Bergel and Schultz, 1971; O'Rourke, 1982), which can modify, depending on the state of the wall muscle, arterial properties. This is possible because the smooth muscle is strong enough to alter the properties of the vessel wall (Nichols and O'Rourke, 1998; Bergel and Schultz, 1971; Barra et al., 1993). In 1902, Bayliss had already proposed that contraction was the reaction of arterial wall smooth muscle to stretching (Bayliss, 1902).
Another important characteristic of smooth muscle is that its tone depends on blood viscosity, blood flow and hematocrit values (Melkumyants et al., 1989; Pohl et al., 1986).
Arterial wall mechanical properties are modulated by neural, myogenic, humoral and local factors. One of the regulators that comes into play is the endothelium, which happens to be involved, together with the smooth muscle, in the functioning of the arterial wall (Schretzenmayr, 1933; Furchgott and Zawadzki, 1980; Pohl et al., 1986).
The physiological role of the endothelial lining may improve the efficiency of the ventricular-arterial coupling energy balance by maintaining the degree of arterial wall viscosity (Boutouyrie et al., 1997). The dilator response of conduit arteries to an augmentation of blood flow was observed more than 70 years ago, and flow-dependant dilation has been suggested as the principal response in a variety of physiological vascular adaptations, such as collateralization and long-term diameter adaptation to increased flow loads (Schretzenmayr, 1933; Furchgott and Zawadzki, 1980). Therefore, large arteries accommodate changes in blood flow by increasing their internal diameter. Such a flow-dependent dilatation represents a fundamental mechanism that opposes neurogenic and myogenic vasoconstriction.
The model mentioned above allows evaluation of arterial wall properties from a dynamic perspective that involves a more complete analysis of physiological and/or pharmacological influences than just a bunch of static indexes.
IV. CIRCULATING LOOP
In the following lines we will describe an apparatus that allows to perform experimental in vitro sessions with conduits submitted to physiological ranges of pressure, diameter, stretching rate and blood hematocrit values. See Fig. 3.
The circulating loop, designed to measure arterial, venous and vascular prostheses' diameter and pressure signals, consists of a pneumatic pump coupled to a perfusion line made of polyethylene and silicon. An organ chamber, a resistant modulator and a reservoir are interposed in between the perfusion line in the order written.
The pneumatic device (Jarvik model 5, Kolff Medical; Salt Lake City, UT) is composed of an input valve, an output valve and two chambers separated by a mobile diaphragm. It is driven by a Utah heart driver, which provides the desired heart rate, pressure values, and length of the systolic and diastolic period for each cycle. When the electrically powered generator propels air out of the pneumatic pump, the input valve opens and the output valve closes, thereby allowing the inflow of blood. Depending on the heart rate chosen, the corresponding time will elapse until the Utah driver infuses air into the pneumatic pump. This, in turn, closes the input valve and opens the output valve, allowing the passage of blood into the perfusion line, where the resistant modulator allows fine adjustments before the fluid reaches the reservoir. Next to the pneumatic pump, is the organ chamber, which contains Tyrode's solution kept at 37oC with a pH of 7.4 bubbled with 100% oxygen. Therefore, the conduit under study remains immersed in the Tyrode's solution (Cabrera Fischer et al., 2002).
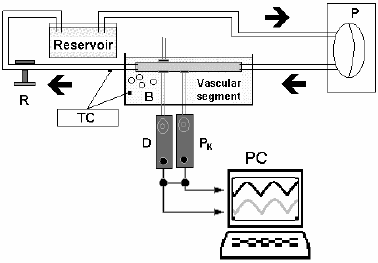
Figure 3. Circulating loop showing a pneumatic pump (P) and a perfusion line with an organ chamber, a resistance modulator (R) and a reservoir. Tyrode's solution is oxygenated through bubbles (B) and thermally controlled (TC). Pressure (Pk) and Diameter (D) are obtained using a solid transducer and ultrasonic crystals. Both signals are stored in a personal computer (PC).
When a biological specimen is analyzed (vein or artery), an in vivo measurement of its length is performed (generally 5 to 7 cm) and two suture references are placed in the adventitia tissue of the vessel. Afterwards, the specimen is mounted in the organ chamber preserving the same in vivo measured length.
Inside the organ chamber a Konigsberg P7 or P2.5 microtransducer (1200 Hz frequency response) is positioned in the conduit to measure intraluminal pressure. This transducer is previously calibrated with a mercury manometer.
To measure diameter of the conduit under study and its changes, a pair of ultrasonic crystals (5 MHz, 2 mm diameter) are employed. Both are attached to the conduit and connected to a Sonomicrometer (Triton Technology Inc. 100 Hz frequency response). The Sonomicrometer converts the transit time of the ultrasonic signal of 1580 m/s into distance. This is observed on the screen of an oscilloscope (Tektronix 465B) to confirm optimal signal quality.
The flow in the perfusion line is monitored with an ultrasonic flowmeter developed for animal use (Model T206, Transonic Systems Inc., 16A/20A Probes, Ithaca, New York, USA).
A similar instrumentational procedure is carried out to be able to measure ePTFE hemodynamic parameters.
This circulating loop also allows to perform changes in blood viscosity. The reservoir is used to provide different levels of hematocrit values within a wide range. The animal blood is centrifuged and the desired red blood concentrate is added to the plasma contained in the reservoir. Viscosity of ovine blood samples (2 ml), anticoagulated with EDTA (1.5 mg/ml), is measured by using a rotational viscometer (LVDT-II + Digital Viscometer, Brookfield, Stoughton, MA) at a shear-rate range of 0.6-200 s-1. Blood viscosity is sampled with the viscometer at a rate of 2 s and those values with a coefficient (defined as the ratio of standard deviation and mean values, expressed as a percentage) of variation >2% are discarded. It is possible to produce different levels of blood hematocrit, aggregation and disaggregation to change blood viscosity.
Figure 4. In vitro pressure-diameter signals obtained in an ovine femoral artery (A) and a jugular vein (B) are shown in the left side. Intraluminal pressure is measured in mmHg (left measurement units) and outer diameter in mm (right measurement units). Both signals are plotted obtaining a pressure-diameter loop (right side).
The circulating loop also allows to perform measurements on de-endothelized arteries, obtained by repeated gentle rubbing with a partially inflated 7-Fr Fogarty embolectomy catheter, which is infused with 2cm of saline solution. The pressure in the catheter rises to 125-130 mmHg; the mean arterial systolic pressure level observed in in vivo condition. To assure the integrity of the arterial wall structure, it is necessary to stay alert for changes in maximum balloon diameter because this value has to be lower than that of the in vivo systolic external diameter to preserve the wall structure.
In a typical experiment, pressure and diameter signals are displayed on a PC monitor and digitized using a specific program manufactured in our laboratory. See Fig. 4.
Finally, as already explained, the circulating loop can change its frequency, also called stretching rate. This is very useful to simulate not only normal physiological values but also pathological ones. The same happens with flow rates, which can vary depending on the physiology of the subject or animal under study (Cabrera Fischer et al., 2002).
That is to say, that each specimen is submitted to the same hemodynamic parameters allowing an isobaric study, if required. See Fig. 4.
V. DATA ANALYSIS
As was described above, pressure and diameter signals are digitized using a specific program manufactured in our laboratory. These samples are recorded and stored for later analysis. The following parameters are calculated using a Borland C++ Software.
The value of vessel wall thickness is quantified as the difference between external radius (re) obtained through ultrasonic measurements and the internal radius (ri) calculated as:
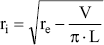 | , (1) |
where V is the volume and L the length of a given vessel segment. The V is calculated by weighing each segment and assuming a tissue density of 1.066 g/ml (Armentano et al., 1995a).
Dynamic changes in diameter of vessels and vascular prostheses are better characterized by the strain (ε) and calculated as:
ε = R/Ro, (2)
where Ro is the nonstressed midwall radius and R is the midwall radius obtained as:
R = (re + ri)/2, (3)
where re, which is obtained by ultrasonic measurements, and ri are the external and internal radius, respectively.
Prostheses, veins and arteries are continuously submitted to an internal blood pressure that determines different levels of wall stress (σ) (Armentano et al., 1995a; Barra et al., 1993). The circumferential stress is calculated as:
 | , (4) |
where P is the internal pressure.
We developed a method to study vascular wall viscoelasticity using pressure-diameter loops or stress-strain loops (Armentano et al., 1995a, b; Barra et al., 1993; Cabrera Fischer et al., 1991). To obtain values of elasticity and viscosity we must take into account the following equation:
 | , (5) |
where Einc is the incremental elastic modulus, η is the viscous modulus and dε /dt is the first derivative of the strain with respect to time. In this formula (Kelvin-Voigt model) the inertial component has been neglected due to the relatively low wall mass of vessels and vascular prostheses (Milnor, 1982; Dobrin and Canfield, 1973; Bauer et al., 1979). See Fig. 2.
The equation presented above (Eq. 5) is a first order linear non-homogeneous equation, which can be transformed to the frequency domain. In fact, Hardung (1952) was the first to describe the viscoelasticity of blood vessels by a complex expression:
| E' = Einc + jωη, | (6) |
where E' is the complex viscoelastic modulus, its real part (Einc) is redefined as the storage modulus and its imaginary part, the loss modulus (ωη), composed by the angular frequency (ω) and (η). (Bergel, 1961; Milnor, 1982; Hardung, 1952). The quotient between imaginary and real part is related to the ratio between energy dissipation (viscous) and energy stored (elastic). The method carried out to obtain the real part of E' (Einc) consists in progressively decreasing the hysteresis loop until it vanishes. It is called the criterion of disappearance of the hysteresis and is useful because Einc is purely elastic, i.e., no hysteresis is involved in its value (Armentano et al., 1995b). See Fig. 5.
The value of the imaginary part of E' is increased by iteration until the minimal hysteresis area that preserves the clockwise direction of the pressure-diameter graph is achieved (Armentano et al., 1998). This value will be the optimal for η.
The incremental elastic modulus (Einc) is a useful tool to quantify the elastic behavior of arteries, veins or vascular prostheses (like ePTFE). To calculate the Einc, an analysis of the pressure-diameter loops is mandatory and its formulae can be expressed as:
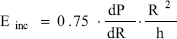 | , (7) |
where dP/dR is the derivative of the pressure-radius loop and h is the wall thickness (Wesly et al., 1975). Einc was always calculated from dP/dR at 93 mmHg pressure (isobaric analysis). See Fig. 5.
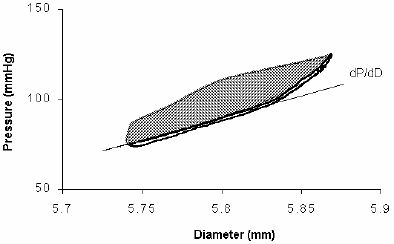
Figure 5. Femoral Pressure-Diameter relationship (thin line) involves the elastic, viscous and inertial properties conforming a hysteresis loop. Considering that the inertial modulus is equal to zero, the viscous modulus value was increased thereby reducing the hysteresis area to a minimum while maintaining the clockwise course of the loop (thick line). Note that the remaining area corresponds to the onset of the loop. The purely elastic pressure-diameter (thick line) is coincident with the pressure-diameter diastolic phase. The Einc was derived from the first derivative of P respect to D (see text).
Both η and Einc are expressions of the arterial buffering behavior. Consequently, it is convenient to include other indexes related to the other main component of the arterial function, which is to serve as a conduit.
The pulse wave velocity is theoretically calculated through the Moens-Korteweg equation.
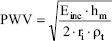 | , (8) |
where Einc is the incremental elastic modulus, ri is the internal radius, ρt is the density of the wall's tissue (ρt = 1.06g/cm3) and hm is the medium parietal width, calculated as the difference between the ri (internal radio) and re (external radio) mean values measured along a complete arterial loop cycle.
The characteristic impedance (ZC) is a useful parameter to evaluate the local arterial conductivity. It is quantified with the Water-Hammer equation:
 | , (9) |
where CSA is the cross sectional area (assuming a cylindrical vascular geometry, CSA=πri2, being ri is the internal radius) and ρs is the blood density (ρ=1.06 g/ml).
The Peterson modulus is a tool for measuring the stiffness of the conduit. It is calculated according to the following equation:
 | , (10) |
where SP and DP are the systolic and diastolic pressures, respectively, and SD and DD are the systolic and diastolic vascular diameter respectively (Peterson et al., 1960). The Peterson Modulus is a useful tool used as an index of arterial stiffness that can be non-invasively obtained in humans.
The Beta stiffness index (E) is another parameter that quantifies the rigidity of the parietal wall. It is calculated as (Nichols and O'Rourke, 1998):
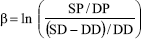 | , (11) |
where ln is the natural logarithm, SP and DP are systolic and diastolic blood pressures and SD and DD are systolic and diastolic diameters, respectively.
The cross sectional compliance (CCS) represents the compliance per area instead of per volume. It is calculated as:
 | , (12) |
where CSAS and CSAD are the systolic and diastolic cross sectional area of the tubular conduit, respectively. SP and DP are the systolic and diastolic pressure, respectively.
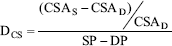
The cross sectional distensibility evaluates the distensibility per area of tubular conduit. It is calculated as:
 | (13) |
where CSAS and CSAD are the systolic and diastolic vascular cross sectional area, respectively. SP and DP are the systolic and diastolic pressure, respectively.
VI. RESULTS
Once the artery, vein or ePTFE is placed in the specimen chamber, the segment is allowed to equilibrate for a period of 15 minutes under a steady state of flow (150 ml/min) and a mean pressure of 93 mmHg, at a stretching rate of 110 beats/min.
In Table 1 we reproduced the hemodynamic values measured in ovine femoral arteries, jugular veins and ePTFE conduits.
TABLE 1: HEMODYNAMICS MEASUREMENTS.
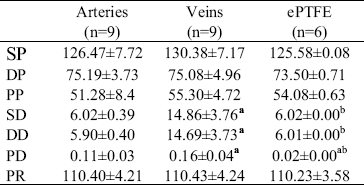
All vessels are submitted to specific hemodynamic parameters similar to those observed in arteries. SP, DP and PP: systolic, diastolic, and pulse pressure (in mmHg), respectively. SD, DD, and PD: systolic, diastolic, and pulse diameter (in mm), respectively. PR: pumping rate (bpm). Values are mean±SD. P values determined by ANOVA + Bonferroni test. (a) with respect to artery: P<0.05. (b) with respect to vein: P<0.05.
As can be seen in Table 1, values obtained of pumping rate, and systolic, diastolic and pulse pressure showed non-significative differences among arteries, veins and ePTFE conduits (P>0.05).
In Table 2 we reproduced the values calculated from measurements in ovine femoral arteries, jugular veins and ePTFE conduits. As can be seen, calculated indexes for ePTFE conduits always showed statistically significative differences with respect to arteries and veins (P<0.05). All calculated indexes for veins, except for pulse wave velocity, showed significative differences with respect to arteries (P<0.05).
TABLE 2: MECHANICAL PARAMETERS
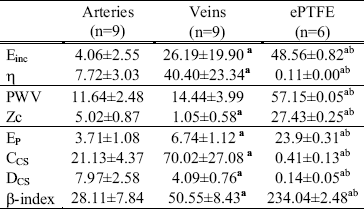
Einc: incremental elastic modulus (107 dyn/cm2). K: viscous modulus (105 dyn.s/cm2). EP: Pressure-strain elastic modulus (106 dyn/cm2). PWV: Pulse Wave Velocity (m/s). CCS and DCS: cross-sectional compliance (10-5 cm2/mmHg) and distensibility (10-4 mmHg-1), respectively. Zc: characteristic impedance (104 dyn.s/cm5). E-index: stiffness beta-index. Values are mean±SD. P values determined by ANOVA + Bonferroni test. (a) respect to artery: P<0.05. (b) respect to vein: P<0.05.
VII. FINAL REMARKS
The circulating loop described above is a device that can provide in vitro physiologic waveforms of pressure and diameter signals of arteries, veins and tubular prostheses, like those of the ePTFE. For the device to be reliable, it has to measure arterial and venous pressure and diameter signals similar to those found in vivo. To achieve this goal, the device allows adjustments of heart rate, length of systolic and diastolic period for each cycle, pressure values, peripheral resistance modulator, different line perfusion (material, diameter and length), and hematocrit level resulting in different levels of blood viscosity. Therefore, it will be mimicking or modeling in vivo situations. In vitro pressure values are regulated to match those obtained in vivo, and then the in vivo and in vitro diameter signals are compared, supporting or not the reliability of the circulating loop. Adjustments of these variables allow to obtain systemic intravascular pressure and diameter signals similar to those observed in vivo. See Fig. 4.
Vessels accommodate changes in blood volume by increasing their diameter determining concomitant values of intraluminal pressures. This is also the behavior of vascular grafts, that is to say ePTFE and veins used as arterial prostheses. To characterize this visco-elastic behavior implies the use of indexes in which it is very important to take into account their dimension dependence or independence.
Several models have been used in order to characterize the viscoelastic properties of the arterial wall. Our model is capable to reproduce the beating activity of the heart that has been involved in the smooth muscle tone mediated by several factors. On the contrary there is no flow in other models (Boutouyrie et al., 1997).
The methodology described above has practical applications since it allows the in vitro dynamic characterization of synthetic and biologic vascular prostheses. This is an important issue, since both veins and ePTFE conduits are used as vascular prostheses in humans, where the native arteries have a different viscoelastic property that result in intimal hyperplasia development. This undesired consequence of vascular prostheses was also observed in human stented carotid arteries (Armentano et al., 2004).
In a previous work we proposed the characterization of wall buffering function by means of the wall time constant, obtained as the ratio η/E when a Kelvin-Voigt model represents the vascular wall (Bia et al., 2004). Accordingly, the P-D relationship could be established using the following formula:

where the η/E ratio would characterize the exponential temporal response of diameter due to a pressure change. This ratio, the time constant of the Kelvin-Voigt model or "time retardation", describes the temporal response of the arterial diameter following acute variations of pressure (creep response or relative damping effect). An elevated value of η/E is related with a slow response, suggesting an augmented buffering effect with an increased attenuation of pressure oscillations.
The vascular conduit function was evaluated by means of the local hemodynamic impedance. It was quantified in terms of the characteristic impedance (ZC). ZC is defined as the impedance in the absence of reflected waves and correlates directly with the elastic properties and inversely with the cross-sectional area of the vascular bed according to the Water-Hammer formula (Nichols and O'Rourke, 1998). An increased ZC will determine an augmented impedance against blood flow, resulting in decreased capacity to conduct blood, without decrease in pressure. Therefore, by inverse reasoning, the conduit function could be computed as 1/ZC.
Recently, invasive techniques measuring instantaneous diameter signals (i.e., intravascular ultrasound) have been used to evaluate particular segments of the systemic and pulmonary vasculature. Some of these techniques even allow simultaneous determination of arterial pressure (Nichols and O'Rourke, 1998). Several mechanical indexes can be calculated from diameter, pressure and/or diameter and pressure single systolic and diastolic values. These individual descriptors of the mechanical properties of the wall are conceptually related but not synonymous. Quantitative and qualitative changes in each of these parameters during passive or active states may depend on the geometric, intrinsic, and/or peripheral effects of VSM activation.
Our in vitro results showed, by means of the Einc values, that under pulsatile isobaric systemic ranges of pressure, both vein and ePTFE were stiffer than that of the native femoral artery, and that the jugular vein was not as stiff as the ePTFE. This is coincident with those reported previously (Baird and Abbott, 1977). Similar behavior was also be noted by observing Ep and the others clinical indexes.
The arterial and vein viscous modulus (η), were always higher than that of the ePTFE. The viscous behavior has been demonstrated to be related to vascular smooth muscle (Armentano et al., 1995a, Bia et al., 2004). Accordingly, it is very important to point out that viscous component (η) of ePTFE wall properties (an artificial conduit) is negligible. This original observation has clinical connotations since all conduits analyzed in this study are usually used as vascular prostheses. This is not a minor subject, since the increased elasticity together with the lack of viscosity in the ePTFE might enhance the mechanical mismatch, between the graft and the native artery. In addition the η/Einc ratios were similar for arteries and veins, but these values were always higher than that of the ePTFE. The similar η/Einc observed in arteries and veins, could be related with the higher patency rates obtained when veins are used for arterial bypass.
The characteristic impedance was higher in ePTFE graft, respect arteries and veins. The essential role of the vascular wall on conduit function can be clearly established when comparing ePTFE and arteries. Notice that, in spite of a similar mean diameter, the higher wall stiffness found in ePTFE causes a decrease in the conduit function (higher ZC) with respect to arteries.
We conclude that mechanical wall characteristics of arteries, veins and synthetic prostheses can be in vitro successfully evaluated through dynamic indexes that analyzed both, the elastic and viscous component of the conduit. This is an important approach, since allows a complete mechanical wall characterization under controlled conditions of pressure, flow, frequency and temperature.
ACKNOWLEDGMENT
This work was partially supported by research grants Programa Desarrollo de las Ciencias Básicas (PEDECIBA), and DINACYT Fondo Prof. Clemente Estable #8487, República Oriental del Uruguay. The authors gratefully acknowledge the technical assistance of Mr. Elbio Agote.
REFERENCES
1. Armentano, R.L., J.G. Barra, J. Levenson, A. Simon and R.H. Pichel, "Arterial wall mechanics in conscious dogs: assessment of viscous, inertial, and elastic moduli to characterize the aortic wall behavior", Circ Res, 76, 468-478 (1995a). [ Links ]
2. Armentano, R.L., J.L. Megnien, A. Simon, F. Bellenfant, J.G. Barra and J. Levenson, "Effects of hypertension on viscoelasticity of carotid and femoral arteries in humans", Hypertension, 26, 48-54 (1995b). [ Links ]
3. Armentano, R.L., S. Graf, J.G. Barra, G. Velikovsky, H. Baglivo, R. Sánchez, A. Simon, R.H. Pichel and J. Levenson, "Carotid wall viscosity increase is related to intima-media thickening in hypertensive patients", Hypertension, 31, 534-539 (1998). [ Links ]
4. Armentano, R.L., E.I. Cabrera Fischer, D. Bia Santana and D. Craiem, "Wall viscosity of the stented arteries", Stroke, 35, 2013(2004). [ Links ]
5. Baird, R.N. and W.M. Abbott, "Elasticity and compliance of canine femoral and jugular vein segments", Am J Physiol, 233(1), H15-H21 (1977). [ Links ]
6. Barra, J.G., R.L. Armentano, J. Levenson, E.I. Cabrera Fischer, R.H. Pichel and A. Simon, "Assessment of smooth muscle contribution to descending thoracic elastic mechanics in conscious dogs", Circ Res, 73, 1040-1050 (1993). [ Links ]
7. Barra, J.G., J. Levenson, R.L. Armentano, E.I. Cabrera Fischer, R.H. Pichel and A. Simon, "In vivo angiotensin II receptor blockade and enzyme inhibition on canine aortic viscoelasticity", Am J Physiol, 272, H859-H868 (1997). [ Links ]
8. Bauer, R.D., R. Busse, A. Schabert, Y. Summa and E. Wetterer, "Separate determination of the pulsatile elastic and viscous forces developed in the arterial wall in vivo", Pflügers Arch, 380, 221-226 (1979). [ Links ]
9. Bayliss, W.M., "On the local reactions of the arterial wall to changes of internal pressure", J Physiol Lond, 28, 220-231 (1902). [ Links ]
10. Bergel, D.H., "The dynamic elastic properties of the arterial wall", J Physiol, 156, 458-469 (1961). [ Links ]
11. Bergel, D.H. and D.L. Schultz, "Arterial elasticity and fluid dynamics", Prog Biophys Mol Biol, 22, 1-36 (1971). [ Links ]
12. Bia, D., J.C. Grignola, R.L. Armentano and F.F. Gines, "Improved pulmonary artery buffering function during phenylephrine-induced pulmonary hypertension", Mol Cell Biochem, 246(1-2), 19-24 (2003). [ Links ]
13. Bia, D., R. Armentano, D. Craiem, J. Grignola, F. Gines, A. Simon and J. Levenson, "Smooth muscle role on pulmonary arterial function during acute pulmonary hypertension in sheep", Acta Physiol Scand, 181(3), 359-366 (2004). [ Links ]
14. Boutouyrie, P., Y. Bézie, P. Lacolley, P. Challande, P. Chamiot-Clerc, A. Benetos, J.F. Renaud de la Faverie, M. Safar and S. Laurent, "In-vivo/in-vitro comparison of rat abdominal aorta wall viscosity. Influence of endothelial function", Arterioscler Thromb Vasc Biol, 17, 1346-1355 (1997). [ Links ]
15. Cabrera Fischer, E.I., R.L. Armentano, J. Levenson, J.G. Barra, M.C. Morales, G.J. Breitbart and R.H. Pichel, "Paradoxically decreased aortic wall stiffness in response to vitamin D3-induced calcinosis. A biphasic analysis of segmental elastic properties in conscious dogs", Circ Res, 68, 1549-1559 (1991). [ Links ]
16. Cabrera Fischer, E.I., R.L. Armentano, F.M. Pessana, S. Graf, L. Romero, A.I. Christen, A. Simon and J. Levenson, "En-dothelium-dependent arterial wall tone elasticity modulated by blood viscosity", Am J Physiol Heart Circ Physiol, 282, 389-394 (2002). [ Links ]
17. Clark, J.M. and S. Glagov, "Transmural organization of the arterial media. The lamellar unit revisited", Arteriosclerosis, 5, 19-34 (1985). [ Links ]
18. Dobrin, P.B. and T.R. Canfield, "Series elastic and contractile elements in vascular smooth muscle", Circ Res, 33, 454-464 (1973). [ Links ]
19. Dobrin, P.B., "Mechanical properties of arteries", Physiol Rev, 58(2), 397-459 (1978). [ Links ]
20. Folkow, B. and E. Neil, The viscosity factor; in Folkow B., Neil E. (eds), Circulation, Oxford University Press, 24-35, London (1971). [ Links ]
21. Furchgott, R.F. and J.V. Zawadzki, "The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine", Nature, 288, 373-376 (1980). [ Links ]
22. Giezeman, M.J.M.M., E. VanBavel, C.A. Grimbergen and J.A.E. Spaan, "Compliance of isolated porcine coronary small arteries and coronary-pressure flow relations", Am J Physiol, 267 (Heart Circ Physiol 36), H1190-H1198 (1994). [ Links ]
23. Gow, B.S. and M.G. Taylor, "Measurement of viscoelastic properties of arteries in the living dog", Circ Res, 23,111-122 (1968). [ Links ]
24. Hardung, V., "Vergleichende Messungen der dynamischen Elastizitat und Viskositat von Blutgefassen, Kautschuk und synthetischen Elastomeren", Helv Physiol Acta, 11, 194-211 (1952). [ Links ]
25. Imura, T., K. Yamamoto, T. Satoh, K. Kanamori, T. Mikami and H. Yasuda, "In vivo viscoelastic behavior in the human aorta", Circ Res, 66, 1413-1419 (1990). [ Links ]
26. Melkumyants, A.M., S.A. Balashov and V.M. Khayutin, "Endothelium dependent control of arterial diameter by blood viscosity", Cardiovasc Res, 23, 41-47 (1989). [ Links ]
27. Milnor, W.K., Properties of the vascular wall, Hemodynamics. Williams & Wilkins, pp. 56-96, Baltimore (1982). [ Links ]
28. Nichols, W.W. and M.F. O'Rourke, Properties of the arterial wall: practice, Nichols, W.W. and M.F. O'Rourke (eds), Mc Donald's blood flow in arteries. Theoretical, experimental and clinical principles, Arnold. 73-97, London (1998). [ Links ]
29. O'Rourke, M.F., Physical properties of blood and arteries; O'Rourke MF (ed), Arterial function in health and disease, Churchill Livingstone. 22-33, New York (1982). [ Links ]
30. Peterson, L.H., R.E. Jensen and J. Parnell, "Mechanical properties of arteries in vivo", Circ Res, 8, 622-639 (1960). [ Links ]
31. Pohl, U., J. Holtz, R. Busse, E. Bassenge, "Crucial role of endothelium in the vasodilator response to increased flow in vivo", Hypertension, 8, 37-44 (1986). [ Links ]
32. Schretzenmayr, A., "Über kreislaufregulatorische Vorgänge an den groβen Arterien bei der Muskelarbeit", Pflugers Arch Ges Physiol, 232, 743-748 (1933). [ Links ]
33. Wesly, R.L.R., R.N. Vaishnav, J.C.A. Fuchs, D.J. Patel and J.C. Greenfield, "Static linear and nonlinear properties of normal and arterialized venous tissue in dog and man", Circ Res, 37, 509-520 (1975). [ Links ]
34. Wetterer, E., R.D. Bauer and R. Busse, New ways of determining the propagation coefficient and the visco-elastic behaviour of arteries in situ; R.D. Bauer and R. Busse (eds), The arterial system-dynamics, control theory and regulation, Springer Verlag pp: 35-47, New York (1978). [ Links ]
35. Wolinsky, H. and S. Glagov, "A lamellar unit of aortic medial structure and function in mammals", Circ Res, 20, 99-111 (1967). [ Links ]
36. Wolinsky, H. and S. Glagov, "Comparison of abdominal and thoracic aortic medial structure in mammals", Circ Res, 25, 667-686 (1969). [ Links ]
37. Wolinsky, H., "Response of the rat aortic media to hypertension", Circ Res, 26, 507-522 (1970). [ Links ]














