Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Latin American applied research
versión impresa ISSN 0327-0793
Lat. Am. appl. res. v.36 n.1 Bahía Blanca ene./mar. 2006
Differences in conduit and buffering function among arteries, venous grafts and synthetic prosthesis: Implicances in the development of intimal hyperplasia
D. Bia1,2, E. I. Cabrera Fischer3,4, Y. Zócalo1, W. Barmak3, F. Pessana3, R. Armentano1,3
1 Depto. Fisiología, Facultad de Medicina, Universidad de la República, Montevideo, Uruguay, CP:11800 (dbia@fmed.edu.uy)
2 ESFUNO. Facultad de Enfermería, Universidad de la República, Montevideo, Uruguay, CP:11300
3 Universidad Favaloro, C1078AAI, Buenos Aires, Argentina (fischer@favaloro.edu.ar)
4 Member of the Research Career, CONICET, Buenos Aires, Argentina.
Abstract — Usually vascular surgeons consider the use of vein grafts as the materials of first choice for bypass or reconstruction of small arteries, while synthetic prosthesis (i.e. polytetrafluoroethylene, ePTFE) plays a secondary role in this matter. However, the causes for the superior performance of vein grafts respect ePTFE remain unclear. Our aim was to compare the conduit (CF) and buffering function (BF) of arteries, with those of vein grafts and ePTFE. In vitro pressure (Konigsberg) and diameter (Sonomicrometry) were measured in ovine arteries and veins, and ePTFE prosthesis, under isobaric and physiological pressures levels. From stress-strain relationship the Kelvin-Voigt time constant was calculated to quantify the BF. The CF was evaluated as 1/ZC, where ZC is the characteristic impedance. Vein graft CF and BF were more similar to native arteries than those of ePTFE prosthesis. Consequently, the higher performance of vein grafts could be related with their superior matching with arteries.
Keywords — Vascular Prostheses. Conduit Function. Buffering Function. Intimal Hyperplasia.
I. INTRODUCTION
The arteries conduit function (CF) allows the distribution of blood to different tissues, while their buffer function (BF) is responsible for the smoothing of pressure and flow pulsatility determined by the intermittent ventricular ejection (Nichols and O'Rourke, 1998; Bia et al., 2003; 2004; 2005a). Both functions are related and depend on the geometrical and mechanical properties of the arteries, which in turn are determined by passive (elastin and collagen fibers) and active components (smooth muscle cells) of the arterial wall. In this sense, its dynamic behavior depends on the viscoelastic individual contribution of each wall constituent and the structural arrangement among them (Bia et al., 2003; 2004; Nichols and O'Rourke, 1998).
In several circumstances in which an arterial segment is altered, an arterial bypass or reconstruction is performed, in order to restore the functional capability. Looking for improving the arterial substitute patency rate, there have been described several conditions that the ideal graft should fulfill. Related to this, taking in to account that the mechanical and/or impedance mismatch between the native artery and the alternative conduit may induce graft failure, it is accepted that an ideal arterial substitute must share identical viscoelastic and geometrical properties with the native artery (Tai et al., 1999). Therefore, in theoretical terms, an autologous artery would be the ideal arterial substitute. However, except in certain limited situations, it is difficult to obtain an autologous artery of adequate length and size without the sacrifice of an organ or essential part of the body.
Several synthetic prosthesis have been employed, and nowadays, among the most widely used are expanded polytetrafluoroethylene (ePTFE) prosthesis. However, occlusion rates of ePTFE when used in small or medium size arterial reconstruction are high. For instance, patency rates for 175 ePTFE femoropopliteal bypasses were 62% at 35 months (Veith et al., 1980). Better results have been obtained with venous grafts. So, the use of autologous veins (mainly the human great saphenous vein) is the first choice in bypass or reconstruction of medium and small arteries, while non-biological prosthetic materials (i.e. ePTFE) assume a secondary role in this position (Dardik and Howard, 1999).
The causes of the superior performance of veins respect to the ePTFE, in the medium and small size arteries' reconstruction, remain to be elucidated (Cabrera Fischer et al., 2005; Norberto et al., 1995; Kissin et al., 2000). The main cause of ePTFE graft failure within a short period of time is the development of intimal hyperplasia (IH) in the distal anastomosed artery (Vijayan et al., 2002; Echave et al., 1979), causing outflow stenosis of prosthetic bypass and tissue ischemia. The mechanical mismatch between the ePTFE and the native artery is considered a major cause of intimal hyperplasya and graft failure (Dardik and Howard, 1999).
The pathogenesis of this lesion is described as proliferation of smooth muscle cells of the arterial media and their migration into the intima, in response to injury at the junction of the graft and the native artery. Over the past years, IH in the bypassed or reconstructed arteries has been extensively studied; however the mechanism through which it develops has not been exactly resolved (Dardik and Howard, 1999). It has been described that early failure, secondary to IH, is merely a consequence of a thrombotic process; posterior obstruction involves an intimal hyperplastic development (Tsui and Dashwood, 2002). In the latter process, smooth muscle cells belonging to the tunica media, migrate through the elastic laminae to the tunica intima releasing a protein matrix. This produces an intimal thickening and is the beginning of the obstruction (Tsui and Dashwood, 2002). This IH, as sequelae of a thrombus organization, determines changes that resemble closely a non-thrombotic genesis (Trubel et al., 1995). In addition, the IH has been related with the trauma during vein harvesting; consequently, the role of handling would be very important in avoiding graft occlusion (Seifalian et al., 2002).
Moreover, two other main factors that affect the development of IH are compliance and wall shear stress mismatch between the native artery and the graft (Trubel et al., 1995; Cabrera Fischer et al., 2005). Since it is very difficult to isolate compliance mismatch from other intervening factors, the direct relationship between compliance mismatch and IH has been confounding. To start with, shear stress and flow rate are linearly correlated, such that a higher flow rate will induce a higher shear stress and vice versa (Tanaka et al., 1996). In turn, shear stress is believed to halter the movement of smooth muscle cells (migration to the intima layer) (Loth et al., 2002). In particular, localized low wall shear stress has been associated with the development of IH, making its first appearance along the suture line and migrating onto the native vessel and graft (Loth et al., 2002; How et al., 2000; Giddens et al., 1993). Therefore, it can be said that there is mounting evidence that flow hemodynamic disturbances induce IH (Loth et al., 2002). One reason for this pathologic behavior might be the deterioration of the endothelium as a consequence of disturbed flow that produces vortices (Vijayan et al., 2002).
Finally, with independence of the mechanism that determine the genesis, IH during high pressure conditions has been stated as an adaptive and natural mechanism to maintain circumferential wall stress, and recently to maintain wall strain, within a normal and uniform level (Dardik and Howard, 1999; Dobrin, 1995). This behavior is called vessel remodeling. Our group has showed that the arterial wall remodeling (i.e. hypertrophy of medial smooth muscle cells) in hypertensive patients occurs with wall viscosity and inertia increase (Armentano et al., 1998; Gamero et al., 1999). This allows not only the stress reduction, but also keeping the wall buffering or filtering function close to the normotensive patients' levels (Armentano et al., 2004). For this mechanism, the arterial system appears to preserve the BF, in order to prevent deleterious high frequency components to damage wall constituents. In the same way, the veins grafts wall remodeling (i.e. intimal and/or medial hyperplasia) (Dobrin, 1995; Jacot et al., 2004; Cabrera Fischer et al., 2005), after interposition in the arterial system could be analyzed as a protective mechanism that reduces the wall stress (Dobrin, 1995), as well as, increases the BF capability maintaining the vein wall buffering performance as arterial segments, in spite of high levels of pressure. In addition, in hypertensive patients, an increase in the arterial internal diameter ("arterial dilatation") has been reported (Armentano et al., 1998). Possibly, the arterial dilatation could be interpreted as an adaptative mechanism to maintain elevated the arterial conduit function, despite of the peripheral resistance increase.
Several groups have investigated and developed techniques to improve ePTFE arterial by-pass results (Kreienberg et al., 2002). Some of these studies showed that the interposition of a vein conduit or segment, known as "vein cuff", between the ePTFE and the distal artery increased the by-pass patency rates (Piorko et al., 2001; Miller et al., 1984). About this, we have demonstrated previously significant quantitative inhibition of IH formation at outflow anastomoses by vein cuff interposition between ePTFE prosthesis and femoral arteries in an ovine model (Cabrera Fischer et al., 2005). It is suggested that vein cuff could reduce the IH, by reducing the mechanical mismatch between the ePTFE prosthesis and the native artery. However, this important topic remains unclear. Additionally, despite of the fact that there are studies that suggest that the venous grafts' major patency rates in small and medium size arteries reconstruction could be related to their biomechanical properties (Dardik and Howard, 1999), until now, there are not studies evaluating and comparing the functional performance of native arteries, vein grafts and synthetic prosthesis, under identical arterial hemodynamic conditions.
Therefore, it remains to be explained if there are differences in arterial and grafts wall behavior during arterial hemodynamic condition; differences that should be taken into account during graft selection.
In this context, our aim was to evaluate and compare the CF and BF of native arteries, with those of veins grafts and ePTFE prosthesis. In addition, we discuss several biomechanical aspects that could be related with the genesis of IH. Our hypothesis was that the vein grafts or cuffs reduce the vascular CF and BF mismatch between the arterial substitute and the native artery, diminishing the mechanical injury to the distal arterial wall.
II. METHODS
A. Surgical procedure
All experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
Nine adult healthy male Merino sheep ranging in weight from 25 to 35 kg were used in this study. All animals were vaccinated and treated for skin and intestinal parasites. During 20 days before surgery they were appropriately fed, watered, and assessed for adequate clinical status. Each sheep was anaesthetized with intravenous administration of pentobarbital (35 mg/kg). The animals were mechanically ventilated.
The distal descending aorta (DDA), and the right common carotid artery (CA), external jugular vein (JV) and femoral vein (FV) were dissected simultaneously in each animal. Segments of 6 cm of DDA, CA, JV and FV, were accurately measured with a caliper and marked with two suture stitches. Afterwards, they were excised and immersed in Tyrode solution, and were destined to in vitro mechanical study. At the end of the surgery, the animals were sacrificed with an overdose of sodium thiopenthane followed by potassium chloride.
B. In vitro study: mechanical test
To perform the mechanical tests, each vascular segment was non-traumatically mounted (at in vivo length) on specifically designed cannulas of the flow circuit loop, and immersed and perfused with a thermally regulated (37o C) and oxygenated Tyrode's solution, with pH= 7.4 (Cabrera Fischer et al., 2002; 2005). A full description of this device (named in vitro system or circulation mock, Fig. 1) has been published previously (Bia et al., 2005b).
Each vessel was instrumented with a pressure microtransducer (1200 Hz frequency response, Konigsberg Instruments, Inc., Pasadena, CA, USA) inserted in the vessels through a stab wound. Each pressure transducer had been previously calibrated using a mercury manometer. To measure vein or arterial external diameter a pair of ultrasonic crystals (5MHz, 2 mm diameter) was sutured to the adventitia of the vessels. The transit time of the ultrasonic signal (1580 m/s) was converted into distance by means of a Sonomicrometer (1000 Hz frequency response, Triton Technology Inc. San Diego, CA, USA). Optimal positioning of the dimensional gauges was assessed by an oscilloscope (model 465B, Tektronix).
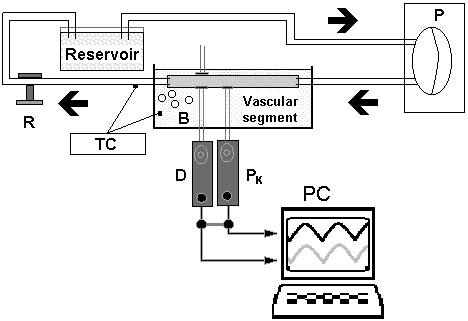
Figure 1. Circulating loop showing a pneumatic pump (P) and a perfusion line with an organ chamber, a resistance modulator and a resevoir. Tyrode's solution is oxigenated through bubles (B) and thermally controlled (TC). Pressure (PK) and Diameter (D) are obtained using a solid tranducer and ultrasonic crystals. Both signals are stored in a personal computer (PC) (Fore more details see reference, Bia et al., 2005b).
Furthermore, seven ePTFE grafts of 6 mm diameter, thin wall (Gore-Tex Vascular graft, W.L.Gore & Associates, Inc., Flagstaff, Arizona, USA) and 6 cm long, were mounted on the in vitro system described above. In order to characterize its mechanical behavior, the ePTFE grafts were instrumented with a pressure microtransducer and a pair of ultrasonic crystals; using the same procedure that was used for DDA, CA, JV and FV. To ensure minimal transmural solution loss and the subsequent decrease of intraluminal pressure, grafts were subjected to a preclotting procedure (Tai et al., 2000). To this end, the graft was perfused several times with 10 ml of fresh sheep blood until no fluid loss was observed. Afterwards, all grafts were washed in normal Tyrode solution.
Once placed in the organ chamber, the vascular and ePTFE segments were allowed to equilibrate for a period of 10 minutes.
C. Experimental protocol and data acquisition
After the equilibration time, a similar protocol was followed for all segments, in order to characterize their mechanical properties.
The pneumatic device of the circulation mock, was regulated by an air supply machine that allowed fine adjustments of heart rate, pressure values and waveforms. Recordings were done submitting the segments to a stretching rate of ~110 cycles/min, an intravascular pulse pressure of ~50 mmHg and mean pressure of ~90 mmHg. In all cases the pump, and tubing resistance were regulated so as to generate adequate pressure waveforms (Bia et al., 2005b).
Vascular and ePTFE segments pressure and external diameter signals were displayed in real time, digitized every 5 ms and digitally stored for later analysis. Approximately 20-30 consecutive beats were sampled and analyzed under steady-state condition.
D. Data analysis
Buffer function
For each segment, to calculate the arterial elastic and viscous modulus the arterial stress-strain loops were constructed (Bia et al., 2005b; Armentano et al., 1995; Barra et al., 1993). The incremental elastic modulus (EINC) and the viscous modulus (η ) were calculated using a Kelvin-Voigt viscoelastic model (spring-dashpot) accordingly with previous works (Bia et al., 2003; 2004; 2005a).
The buffer function (BF) was obtained by analyzing the temporal response of the arterial strain to an abrupt increase of stress, performed via a Kelvin-Voigt model of the arterial wall (Bia et al., 2004; 2005a). Accordingly, the σ -ε relationship was established using EINC and η
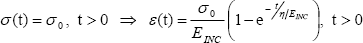
where the η /EINC ratio would characterize the exponential temporal response of strain due to a stress change. This ratio, the time constant of the Kelvin-Voigt model or "time retardation" (Westerhof and Noordergraaf, 1970), characterizes the intrinsic capacity of the material to cushion the stress or pressure exerted over its surface. As the material involved in this case is the arterial, venous or ePTFE wall, the conduit wall BF from Kelvin-Voigt model was calculated as (Bia et al., 2003; 2004; 2005a):
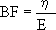
An elevated value of BF is related with a slow response, suggesting an augmented buffering effect with an increased attenuation of stress or pressure oscillations (Bia et al., 2004; 2005a; 2005b).
Conduit function
The conduit function (CF) was evaluated by means of the local hemodynamic impedance, as was previously reported (Bia et al., 2005a). It was quantified in terms of the characteristic or local impedance (ZC), following Water Hammer equation (Nichols and O'Rourke, 1998):
ZC = PWV*ρB/CSAM
where ρ B is the blood density (ρB=1.06 g/mL), CSAM is the mean cross-sectional area (assuming a cylinder geometry for the artery, CSA=π ·Ri2), where Ri is the internal radio, and PWV is the pulse wave velocity. The PWV was calculated theoretically by the Moens-Korteweg equation (Nichols and O'Rourke, 1998):
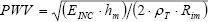
where hm is the mean wall thickness, Rim is the mean internal radius, and ρ T is the wall tissue density. An increased ZC will determine an increase in the resistance against blood flow (Cholley et al., 2001) and consequently a diminished capacity to conduct blood, without a decrease of pressure. Therefore, in this study the conduit function, CF, was analyzed as (Bia et al., 2005a):
CF = 1/ZC
E. Statistical analysis
Hemodynamic and biomechanical parameters were compared using one-way analysis of variance and the Bonferroni t test. Values reported are expressed as mean value ± standard deviation. A p<0.05 threshold was adopted as statistically significant. All statistical analysis were performed using a specific program (Systat 10, Chicago, Illinois, USA).
III. RESULTS.
In Table 1 we reproduced the hemodynamic values measured in ovine arteries and veins, and ePTFE conduits.
Table 1: hemodynamics measurements.
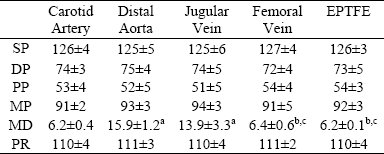
All vessels are submitted to specific hemodynamic parameters similar to those observed in arteries. SP, DP, PP and MP: systolic, diastolic, pulse and mean pressure (in mmHg), respectively. MD: mean diameter (in mm), respectively. PR: pumping rate (bpm). Values are mean ± SD. P values determined by ANOVA + Bonferroni test: a, b, c, and d: p<0.05 with respect to carotid artery, distal aorta, jugular vein and femoral vein, respectively.
Values obtained of pumping rate, and systolic, diastolic, pulse and mean pressure showed non-significative differences among arteries, veins and ePTFE (p>0.05), ensuring an isobaric and isofrequency comparison (Table 1). Note that the external diameter was similar among carotid arteries, femoral veins and ePTFE segments. Additionally, the external diameter, was similar between the distal aorta and the jugular vein.
In Table 2 we reproduced the functional parameters, CF and BF, calculated from measurements in ovine and ePTFE segments. As can be seen, the CF did not show statistical differences between the carotid artery and the femoral vein. Additionally, the CF of the jugular was higher respect the CF of the carotid artery (p<0.05) and lesser respect to the CF of the distal aorta (p<0.05). In contrast the CF of the ePTFE segments was lesser respect the CF of arteries and veins (p<0.01).
The BF of jugular and femoral veins showed statistically significative differences with respect to arteries (p<0.05) and ePTFE conduits (p<0.05). The BF of the ePTFE segments was lesser respect the BF of both, arteries and veins (p<0.01).
IV. DISCUSSION.
The aim of this work was to characterize, and compare the CF and BF of native arteries, with those of vein grafts and ePTFE prosthesis. In addition, we analysed several biomechanical factors that could play important roles in the genesis of IH.
The following discussion focuses on three central aspects of this work.
- Methodological strategy.
- Vascular Grafts Functionality.
- Final Remarks.
Table 2: Functional Parameters

BF: wall buffering function (10-2 s). CF: conduit function (10-4 cm5/dyn.s). Values are mean ± SD. p values were determined by ANOVA + Bonferroni test: a, b, c, and d; p<0.05 with respect to carotid artery, distal aorta, jugular vein and femoral vein, respectively.
A. Methodological strategy
We studied ovine vascular segments taking into account that the ovine cardiovascular system has shown to be an excellent (and widely used) model to study the hemodynamic performance of vascular grafts, due to its similarity to the human cardiovascular system (Kohler and Kirkman, 1999; Cabrera Fischer et al., 2005). In previous works we have employed this model to evaluate the performance of biological and synthetic grafts (Cabrera Fischer et al., 2005; Bia et al., 2005a; 2005b).
We opted for vascular segments, instead of the most used strips or rings (Silver et al., 2003; Mavrilas and Tsapikouni, 2002), because the former are better to reproduce the in vivo hemodynamic conditions, and to preserve the shape and integrity of vascular/graft wall (Dobrin, 1995).
The technique employed to register pressure and diameter with the in vitro circulation mock (Figure 1), has been previously used and validated by our group (Armentano et al., 1995; Cabrera Fischer et al., 2002; Bia et al., 2005b). This methodology, allows accurate and reproducible measurements due to the high frequency and linearity of response of both, dimension gauges and pressure transducers. The 200 Hz sampling rate used in the digitalization of the data was larger than the highest frequency components in the pressure and diameter spectra, enabling signal reconstruction without distortion.
The stress-strain relationship analysis allows the characterization of the vascular wall mechanical behavior (Armentano et al., 1995; Bia et al., 2005; 2005b). This analysis illustrates that the vascular wall has a non-linear, and frequency-dependent mechanical behavior (Armentano et al., 1995; Bia et al., 2005c). Although static analyses (i.e. incremental stress-strain test) allow the evaluation of frequency-dependent mechanical properties (i.e. wall viscosity) (Mavrilas and Tsapikouni, 2002; Silver et al., 2003), an adequate characterization of the functional meaning and contribution of this properties requires dynamic analysis, in which the vascular wall is submitted to conditions similar to real hemodynamic situations. Consequently, in order to adequately characterize the arterial and vein wall mechanical functional behavior, in this work we performed a dynamic analysis of the vessels' wall. To this aim, physiological pressure and diameter waveforms, and arterial pressure and stretch frequency levels were simulated in the mock system (Table 1).
Once used in arterial reconstruction, vein grafts or ePTFE prosthesis, and native artery diameter or strain, may not necessarily be the same. In fact, when a vein is interposed in the arterial system, the blood pressure induced strain in the vessel, makes the vein graft to operate in its high strain region of the pressure-diameter or stress-strain curve (Cabrera Fischer et al., 2005). On the contrary, it has been estimated that the blood pressure induced strain in the ovine femoral artery is between 10-15%, suggesting that the arteries physiologically operate in the low strain region and/or in beginning part of the high strain region of the stress-strain curve (Bia et al., 2003; 2005c). Therefore, an isometric analysis or comparison, whereas biomechanically interesting (Mavrilas and Tsapikouni, 2002; Silver et al., 2003), may not reproduce what does really happen when vein grafts are used in arterial reconstruction. In opposition, when a vein graft is interposed in the arterial system, both native artery and vein and ePTFE graft are subjected to the same vascular pressure and frequency of stimulation, corresponding to that of the arterial system. Therefore, in order to perform an appropriate mechanical characterization and comparison we did an isobaric and isofrequency dynamic study of arteries, veins and ePTFE segments submitted to hemodynamic conditions similar to that of systemic arteries (Armentano et al., 1995; Bia et al., 2005c). To perform this kind of study an in vitro evaluation was compulsory. To our knowledge, similar studies have not been previously reported.
B. Vascular Grafts Functionality
The CF of the arteries, veins and ePTFE segments was evaluated by means of the characteristic impedance (ZC). ZC correlates directly with the elastic properties and inversely with the cross-sectional area of the conduit or vascular bed. An increased ZC will determine an increase in local impedance against blood flow resulting in a decreased capacity to conduct blood (Cholley et al., 2001). Therefore, by inverse reasoning, the CF was computed as 1/Zc.
The CF of the arteries allows the blood transference from the heart to the peripheral vessels. To maintain an adequate level of mean pressure and to minimize ventricular work and wave reflections, low arterial impedance must be presented to the pulsatile blood flow ejected by the heart (Pepine and Nichols, 1982; Morita et al., 2002). The implantation of a rigid graft into the arterial tree −comparable to the introduction of an impedance into an oscillating electrical circuit− diminishes the perfusion efficiency and, in low-flow situations, may lead to further flow stagnation and graft thrombosis (Tai et al., 2000), with concomitant increase in ventricular afterload when the rigid graft is implanted in a large artery location (Morita et al., 2002).
Our results showed that the CF calculated isobarically did not evidenced significant differences between the carotid artery (a small artery) and the femoral vein. Furthermore, the jugular vein showed a higher conduit function respect to the carotid artery. In contrast, the ePTFE conduit showed a lesser CF, that it is to say a higher impedance, respect to all the arteries and veins segments. Consequently, the vein grafts reproduce and/or increase the in vivo conduit performance of the small native arteries. Therefore, at least in theoretical terms, the implantation of a vein grafts into the carotid region would not increase the impedance in the vascular circuit, allowing to maintain the arterial perfusion efficiently, and maintaining the ventricular afterload similar to normal values. In contrast the ePTFE, diminishes the perfusion efficiency, generating non physiological impedance levels.
C. Buffering function
Recently, our group proposed the characterization of wall BF by means of the arterial wall time constant, obtained as the ratio η /EINC when a Kelvin-Voigt model represents the arterial wall (Bia et al., 2005a). A large η /EINC value, associated with a slow response, suggests an enhanced wall buffering function by means of a more pronounced attenuation of the pressure or stress oscillations.
The η /EINC has the elastic modulus as denominator and has a dissipative term as numerator. The elasticity is responsible for the storage capacity of the arterial wall. The energy dissipation is related to the viscous properties of the arterial wall (Bia et al., 2005a) and helps to attenuate traveling pressure pulses along the arteries, and prevents reflected pressure waves from resonating in the arterial system (Shadwick, 1999). In turn, wall elasticity and viscosity influence the propagation and interaction of pressure and flow waves throughout the arterial tree and, consequently, the energetic demands placed on the heart (Shadwick, 1999; Morita et al., 2002). Thus, vascular viscosity and elasticity are not only important determinants of the arterial functions, but also of the whole cardiovascular system performance (Morita et al., 2002).
In addition, the arterial wall BF, that is to say the capability of the arterial wall to store, transmit, and dissipate energy, is key to prevent early mechanical failure or disruption of the arterial wall components. As a law, high frequency vibrations produce structure injures, and the aim of filtering is to reduce accelerated oscillations. The wall energy dissipation avoids the transference of the high frequency components of the pressure wave to vascular diameter, exerting a protective effect on the arterial wall.
In agreement to previous works, we found no differences in the BF between both arterial segments (Bia et al., 2005a). Interestingly, the BF of veins was lesser respect to arteries. This seems that the veins submitted to arterial hemodynamic conditions do not have the "arterial" buffer or filtering capacity, allowing the high frequencies of pressure wave to be present in the diameter signals. This effect of high frequencies, provokes a detriment in the mechanical protection of the vein wall segment (Pontrelli and Rossoni, 2003). This could be related to the recognized frequent progression of the atherosclerotic disease in the vein grafts, and/or with the vein grafts intimal hyperplasia and/or remodeling (Dobrin, 1995; Berceli et al.,1990).
However, the ePTFE segments showed a lesser BF respect arteries and veins. Consequently, if an ePTFE segment were interposed in the arterial system, its lower buffering capacity would provoke a high pressure and/or flow pulsatility in the distal artery. About this, when the incident pulse pressure wave enters the stiff synthetic graft, its amplitude could increase abruptly. Therefore, when the pressure waves arrive to the distal arterial segment, their high pulsatility could provoke a non-physiological wall stress level. The high stress level in the distal anastomosis has been related to the recognized high incidence of the intimal hyperplasia in the distal graft-artery anastomosis (Dobrin, 1995).
D. Final Remarks
At date, it is accepted that, when an arterial segment is replaced, the ideal arterial graft used for arterial bypass and/or reconstruction, must share identical geometric and viscoelastic properties with the host artery (Seifalian et al., 2002). However, the commercially available prosthetic vascular grafts (i.e. ePTFE), are far from that ideal, as our and other authors results showed (Seifalian et al., 2002). The synthetic prostheses are extremely rigid compared to elastic and muscular native arteries (Cabrera Fischer et al., 2005; Tai et al., 2000).
In this work we evaluated the vascular functions – conduit and buffering functions) of arteries, vein grafts and ePTFE prosthesis in order to determinate if functional differences among them could be related with the IH development. Our data showed that the vein graft conduit and buffering performance were more alike to arteries than synthetic prosthesis.
Consequently, the graft functionality could be related with the higher patency rates of vein grafts respect ePTFE prosthesis in small and medium size arteries reconstruction.
ACKNOWLEDGMENT
This work was partially supported by research grants Programa Desarrollo de las Ciencias Básicas (PEDECIBA), República Oriental del Uruguay. The authors gratefully acknowledge the technical assistance of Mr. Elbio Agote.
REFERENCES
1. Armentano R.L., J.G. Barra, J. Levenson, A. Simon and R.H. Pichel, "Arterial wall mechanics in conscious dogs: assessment of viscous, inertial, and elastic moduli to characterize the aortic wall behavior", Circ Res, 76, 468-478 (1995). [ Links ]
2. Armentano R.L., S. Graf, J.G. Barra, G. Velikovsky, H. Baglivo, R. Sanchez, A. Simon, R.H. Pichel and J. Levenson, "Carotid wall viscosity increase is related to intima-media thickening in hypertensive patients", Hypertension, 31, 534-539 (1998). [ Links ]
3. Armentano R.L., D. Bia, D. Craiem, S. Graf, F. Pessana, L. Brandani, H. Baglivo and R. Sánchez, "Smooth muscle energy dissipation is modulated by acei treatment in human hypertension", XIV Fourteenth European Meeting on Hypertension. Paris (France), June 13-17, (2004). [ Links ]
4. Barra J.G., R.L. Armentano, J. Levenson, E.I. Fischer, R.H. Pichel and A. Simon, "Assessment of smooth muscle contribution to descending thoracic aortic elastic mechanics in conscious dogs", Circ Res, 73, 1040-1050 (1993). [ Links ]
5. Berceli S.A., D.P. Showalter, R.A. Sheppeck, W.A. Mandarino and H.S. Borovetz, "Biomechanics of the venous wall under simulated arterial conditions", J Biomech, 23, 985-989 (1990). [ Links ]
6. Bia, D., R.L. Armentano, J.C. Grignola, D. Craiem, Y.A. Zocalo, F.F. Gines and J. Levenson, "The vascular smooth muscle of great arteries: local control site of arterial buffering function?", Rev Esp Cardiol, 56, 1202-1209 (2003). [ Links ]
7. Bia, D., R. Armentano, D. Craiem, J. Grignola, F. Gines, A. Simon and J. Levenson, "Smooth muscle role on pulmonary arterial function during acute pulmonary hypertension in sheep", Acta Physiol Scand 181, 359-66 (2004). [ Links ]
8. Bia, D., J.G. Barra, J.C. Grignola, F.F. Gines and R.L. Armentano, "Pulmonary artery smooth muscle activation attenuates arterial dysfunction during acute pulmonary hypertension", J Appl Physiol., 98, 605-613 (2005a). [ Links ]
9. Bia, D., R.L. Armentano, Y. Zócalo, W. Barmak, E. Migliaro and E. Cabrera Fischer, "In vitro model to study arterial wall dynamics through pressure-diameter relationship analysis", LAAR, 35, 217-224 (2005b). [ Links ]
10. Bia, D., I. Aguirre, Y. Zocalo, L. Devera, E. Cabrera Fischer and R. Armentano, "Regional differences in viscosity, elasticity and wall buffering function in systemic arteries: pulse wave analysis of the arterial pressure-diameter relationship", Rev Esp Cardiol. 58, 167-174 (2005c). [ Links ]
11. Cabrera Fischer, E.I., R.L. Armentano, F.M. Pessana, S. Graf, L. Romero, A.I. Christen, A. Simon and J. Levenson, "Endothelium-dependent arterial wall tone elasticity modulated by blood viscosity", Am. J Physiol Heart Circ Physiol, 282, 389-394 (2002). [ Links ]
12. Cabrera Fischer E.I., D. Bia Santan, G.L. Cassanello, Y. Zócalo, E.V. Craword, R. Casas and R.L. Armentano, "Reduced elastic mismatch achieved by interposing vein cuff in expanded polytetrafluoroethylene femoral bypass decreases intimal hyperplasia", Artificial Organs, 29, 122-130 (2005). [ Links ]
13. Cholley B.P., R.M. Lang, C.E. Korcarz and S.G. Shroff, "Smooth muscle relaxation and local hydraulic impedance properties of the aorta", J Appl Physiol, 90, 2427-2438 (2001). [ Links ]
14. Dardik, H. and H. Greisler, "History of prosthetic grafts", In: Seminars in Vascular Surgery. Vascular grafts: past, present, and future, Editor: Robert B. Rutherford. Guest editors: Howard Greisler, and Herbert Dardik. Published by: W.B. Saunders company, 12, 1-7 (1999). [ Links ]
15. Dobrin, P.B., "Mechanical factors associated with the development of intimal and medial thickening in vein grafts subjected to arterial pressure. A model of arteries exposed to hypertension", Hypertension, 26, 38-43 (1995). [ Links ]
16. Echave, V., A.R. Koornick, M. Haimov and J.H. Jacobson II, "Intimal hyperplasia as a complication of the use of the polytetra fluoroethylene graft for femoral-popliteal bypass", Surgery, 86, 791-798 (1979). [ Links ]
17. Gamero, L., J. Levenson, R. Armentano, S. Graf, L. Brandani, A. Simon, H. Baglivo and R. Sanchez, "Carotid wall inertial index increase is related to intima-media thickening in hypertensive patients", J Hypertens, 17, 1825-1829 (1999). [ Links ]
18. Giddens, D.P., C.K. Zarins and S. Glagov, "The role of fluid mechanics in the localization and detection of atherosclerosis", Transactions ASME, 115, 588-594 (1993). [ Links ]
19. How, T.V., C.S. Rowe, G.L. Gilling-Smith and P.L. Harris, "Interposition vein cuff anstomosis alters wall shear stress distribution in the recipient artery", J Vasc Surg, 31, 1008-1017 (2000). [ Links ]
20. Jacot, J.G., I. Abdullah, M. Belkin, M. Gerhard-Herman, P. Gaccione, J.F. Polak, M.C. Donaldson, A.D. Whittemore and M.S. Conte, "Early adaptation of human lower extremity vein grafts: wall stiffness changes accompany geometric remodeling", J Vasc Surg, 39, 547-55 (2004). [ Links ]
21. Kissin, M., N. Kansal, P.J. Pappas, D.O. DeFouw, W.N. Durán and R.W. Hobson II, "Vein interposition cuffs decrease the intimal hyperplastic response of polytetrafluoroethylene bypass grafts", J Vasc Surg, 31, 69-83 (2000). [ Links ]
22. Kohler, T.R. and T.R. Kirkman, "Dialysis access failure: a sheep model of rapid stenosis", J Vasc Surg, 30, 744-751 (1999). [ Links ]
23. Kreienberg, P.B., R.C. Darling III, B.B. Chang, B.J. Champagne, P.S.K. Paty, S.P. Roddy, W.E. Lloyd, K.J. Ozsvath and D.M. Shah, "Early results of a porspective randomized trial of spliced vein versus polytetrafluoroethylene graft with a distal vein cuff for limb-threatening ischemia", J Vasc Surg., 35, 299-306 (2002). [ Links ]
24. Loth, F., S.A. Jones, C.K. Zarins, D.P. Giddens, R.F. Nassar, S. Glagov and H.S. Bassiouny, "Relative contribution of wall shear stress and injury in experimental intimal thickening at PTFE end-to-side arterial anastomoses", J Biomech Eng, 124, 44-51 (2002). [ Links ]
25. Mavrilas, D. and T. Tsapikouni, "Dynamic mechanical properties of arterial and venous grafts used in coronary bypass surgery", Journal of Mechanics in Medicine and Biology, 2, 1-9 (2002). [ Links ]
26. Miller, J.H., R.K. Foreman, L. Ferguson and I. Faris, "Interposition vein cuff for anastomosis of prosthesis to small artery", Aust N Z J Surg, 54, 283-285 (1984). [ Links ]
27. Morita, S., T. Asou, I. Kuboyama, Y. Harasawa, K. Sunagawa and H. Yasui, "Inelastic vascular prosthesis for proximal aorta increases pulsatile arterial load and causes left ventricular hypertrophy in dogs", J Thorac Cardiovasc Surg, 124, 768-74 (2002). [ Links ]
28. Nichols, W.W. and M.F. O'Rourke, "McDonald's Blood Flow in Arteries", Theoretical, Experimental and Clinical Principles. London, UK: Edward Arnold (1998). [ Links ]
29. Norberto, J.J., A.N. Sidawy, K.S. Trad, B.A. Jones, R.F. Neville, S.F. Najjar, M.K. Sidawy and R.G. DePalma, "The protective effect of vein cuffed anastomoses is not mechanical in origin", J Vasc Surg 21, 558-64 (1995). [ Links ]
30. Pepine, C.J. and W.W. Nichols, "Aortic input impedance in cardiovascular disease", Prog Cardiovasc Dis, 24, 307-318 (1982). [ Links ]
31. Piorko, D., P. Knez, K. Nelson and T. Schmitz-Rixen, "Compliance in anastomoses with and without vein cuff interposition", Eur J Vasc Endovasc Surg, 21, 461-466 (2001). [ Links ]
32. Pontrelli, G. and E. Rossoni, "Numerical modelling of the pressure wave propagation in the arterial flow", Int J Numer Meth Fluids, 43, 651-671 (2003). [ Links ]
33. Seifalian, A.M., A. Tiwari, G. Hamilton and H.J. Salacinski, "Improving the clinical patency of prosthetic vascular and coronary bypass grafts: the role of seeding and tissue engineering", Artificial Organs, 26, 307-320 (2002). [ Links ]
34. Shadwick, R.E., "Mechanical design in arteries", J Exp Biol, 202, 3305-3313 (1999). [ Links ]
35. Silver, F.H., P.B. Snowhill and D.J. Foran, "Mechanical behavior of vessel wall: a comparative study of aorta, vena cava, and carotid artery", Ann Biomed Eng, 31, 793-803 (2003). [ Links ]
36. Tai, N.R., A. Giudiceandrea, H.J. Salacinski, A.M. Seifalian and G. Hamilton, "In vivo femoropopliteal arterial wall compliance in subjects with and without lower limb vascular disease", J Vasc Surg, 30, 936-945 (1999). [ Links ]
37. Tai, N.R., H.J. Salacinski, A. Edwards, G. Hamilton and A.M. Seifalian, "Compliance properties of conduits used in vascular reconstruction", Br J Surg, 87, 1516-1524 (2000). [ Links ]
38. Tanaka, Y., D.P. Schuster, E.C. Davis, A. Patterson and M.D. Botney, "The role of vascular injury and hemodynamics in rat pulmonary artery remodeling", J Clin Invest, 98, 34-42 (1996). [ Links ]
39. Trubel, W., H. Schima, A. Moritz, F. Raderer, A. Windisch, R. Ulrich, U. Windberger, U. Losert and P. Polterauer, "Compliance mismatch and formation of distal anastomotic intimal hyperplasia in externally stiffened and lumen-adapted venous grafts", Eur J Vasc Endovasc Surg, 10, 15-23 (1995). [ Links ]
40. Tsui, J.C.S. and M.R. Dashwood, "Recent strategies to reduce vein graft occlusion: a need to limit the effect of vascular damage", Eur J Vasc Endovasc Surg, 23, 202-208 (2002). [ Links ]
41. Veith, F.J., S. Gupta and V. Daly, "Management of early and late thrombosis of expanded polytetrafluoroethylene (PTFE) femoropopliteal bypass grafts: Favorable prognosis with appropriate reoperation", Surgery, 581-587 (1980). [ Links ]
42. Vijayan, V., F.C.T. Smith, G.D. Angelini, R.A. Bulbulia and J.Y. Jeremy, "External Supports and the Prevention of Neointima Formation in Vein Grafts", Eur J Vasc Endovasc Surg, 24, 13-22 (2002). [ Links ]
43. Westerhof, N. and A. Noordergraaf, "Arterial viscoelasticity: a generalized model. Effect on input impedance and wave travel in the systematic tree", J Biomech, 3, 357-379 (1970). [ Links ]














