Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Latin American applied research
Print version ISSN 0327-0793
Lat. Am. appl. res. vol.36 no.3 Bahía Blanca July/Sept. 2006
Enantioselective extraction of ketoprofen enantiomers using ester alcohol R, R-di-tartarates or S, S-di-tartarates as chiral selector
K. Huang, F. Jiao, S. Liu, X. Li and D. Huang
School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China
jiaofp@163.com
Abstract — Distribution behavior of ketoprofen enantiomers was examined in a two-phase system containing R, R-di-tartarates or S, S-di-tartarates. The influences of different alkyl chain of R, R-di-tartarates or S, S-di-tartarates, concentrations of R, R-di-tartarate, organic solvent and content of methanol on partition coefficient and separation factor were investigated, respectively. The experimental results show that R, R-di-tartarates studied all form more stable diastereomeric complexes with ketoprofen S-enantiomer than with R-enantiomer. The partition coefficients and enantioselectivity generally increase with the addition of length of alkyl chain of alcohol. The concentration of chiral selector and methanol also have bigger influences on enantioselectivity.
Keywords — Enantioselective Extraction. Ketoprofen Enantiomers. Partition Coefficient. Separation Factor. Chiral Selector.
I. INTRODUCTION
Ketoprofen, 2-(3-benzoylphenyl) propionic acid is a nonsteroidal anti-inflammatory drug (NSAID) which has potent inhibitory effects on prostaglandin synthesis. It is commonly used in the treatment of rheumatoid arthritis and osteoarthritis. Therapeutic doses of ketoprofen have proven to be as effective as those of the other commonly used NSAIDs. Ketoprofen is marketed as the racemate although, like the other members of the 2-arylpropanoic acid class, it exhibits enantioselectivity in its action and disposition. Specifically, in vitro experiments proved that the S (+) enantiomer exhibited phar-maco-logical effects, but the R (-) enantiomer was inactive. Furthermore, an in vivo experiment showed that the chiral inversion takes place from R (-) to S (+) with the degree of inversion varying from one animal species to another (Blanco et al., 1998). Various approaches such as diastereomer crystallization (Yoneyoshi et al., 1996; Lukas et al., 1991), kinetic resolution (Antona et al., 2002; Park et al., 1999; Jaeger and Reetz, 1998; Crescenzo et al., 2000; Kato et al., 2000; Jin et al., 2003; Kumar and Jolly, 2001; Madhav and Ching, 2001; Kim et al., 2000; Lui et al., 2000; Shen et al., 2002; Kim et al., 2002a; Choi et al., 2003; Kim et al., 2002b; Xi and Xu, 2005; Liu et al., 2004), asymmetric synthesis (Babin and Whiteker, 1996; Laue et al., 1994; Ramminger et al., 2000; Rossi et al., 1993; Shimizu et al., 1991; Fadel, 1992), chromatography separation (Blanco et al., 1998; Davies, 1997; Boisvert et al., 1997) and preferential crystallization (Eikeren et al., 1997; Manimaran and Potter, 1992) were reported for the production of S-enantiomer of ketoprofen. However, the major limitation of S-ketoprofen production is that the maximum yield is limited to 50 % of the desired enantiomer based on the racemic starting substrate, which consists of equimolar of R-enantiomer and S-enantiomer. The use of enantiomeric technology is only in the separation of the 50 % of the desired S-enantiomer, while the other 50 % of R-enantiomer remained as byproduct. The effort of recovering the 50 % of the byproduct is important in order to achieve the maximum yield of more than 50 %.
To avoid the problems above, extraction with an optically active extractant is a very attractive option (Vladimir et al., 1989, Jerome et al., 2000). Though the selectivity of a single extraction is small, extractions with many stages can be well established. The capacity of extraction is often much greater than that of chromatography etc, extraction can easily be operated continuously.
Diester derivatives of tartaric acid are well known as effective chiral selectors. Because these derivatives are symmetric in C2 axis and two kinds of functional groups, hydroxyl and carbonyl, attached to asymmetric carbons in these derivatives are stereochemically equivalent to those groups attached to other carbons; these structural features are favorable for them to be chiral selectors (Heldin et al., 1991). Ketoprofen has a carboxylic acid group at the chiral center that could participate in additional interactions with the hydroxyl and carbonyl groups of the enantiopure di-tartarates.
In this paper, the distribution behavior of ketoprofen enantiomers was examined in the aqueous and organic solvent of a two-phase systems containing R, R-di-tartarates or S, S-di-tartarates. The effects of different alkyl chain of R, R-di-tartarates, concentration of chiral selector, organic solvent and content of methanol on enantioselectivity (α) and partition coefficient (k) were investigated to find favourable experimental conditions for resolution. As far as we know, there is no report on the enantioselective extraction of ketoprofen enantiomers using R, R-di-tartarates or S, S-di-tartarates as chiral selector.
II. MATERIAL AND METHODS
A. Chemicals
Racemic ketoprofen was purchased from Xunda Group Corporation Pharmaceutical Factory in China (> 98 %). Vancomysin was obtained from Huabei Group Corporation Pharmaceutical in China. For the determination of enantioselectivities different R, R-di-tartarates or S, S-di-tartarates were used, which were prepared in our laboratory from R, R-tartaric acid or S, S-tartaric acid and alcohol, respectively, and were > 98 % (Heldin et al., 1991). Other reagents utilized were all of analytical grade and were purchased from different suppliers. Water was deionized and bidistilled.
B. Analytical Method
Chromatographic studies were performed using a LC-10 AD pump (Shimadzu, Japan), an SIL-10 A injection valve with 20 μl loop, an SPD-10 A UV/VIS spectro-photometer detector (Shimadzu, Japan) at 300 nm. An AT-130 temperature controller (Autoscience, Tianjin, PR, China) was used to control column temperature. A Lichrospher C8 column (200 mm×4.6 mm i.d., 5 μm) was used for analysis. The pH measurement was performed on a pH meter (Orion, model 818, Shanghai, China).
Mobile phases were composed of methanol: 0.25 % triethylamine acetate buffer (50:50) with 3.5 mmol/L vancomysin, at pH 5.5. The column was operated at ambient temperature 20 oC. The flow-rate was set at 0.6 ml/L.
C. Enantioselective Experiments
The aqueous phase was prepared by dissolving a certain amount of ketoprofen in water. To increase the solubility of ketoprofen, the use of amounts of organic solvent of methanol in aqueous phase is especially important, which was proved to be no influencing on experimental results. The organic phase was prepared by dissolving a certain concentration of R, R-di-tartarates or S, S-di-tartarates in organic solvents. In a 10 mL separator/ funnel, 5 mL of the aqueous phase was intensely shaken for some minutes with 5 mL of the organic phase at room temperature. After the two phases were separated, the concentrations of enantiomer R and enantiomer S were measured by HPLC. The partition coefficient can be expressed as k=(Cij-Ceqj)/Ceqj, where Cij is the initial concentration of ketoprofen in aqueous phase and Ceqj is the concentration of ketoprofen in the aqueous phase after equilibration, j is used to denote the enantiomer concerned (R or S). The separation factor α is the ratio of partition coefficients of ketoprofen S to ketoprofen R.
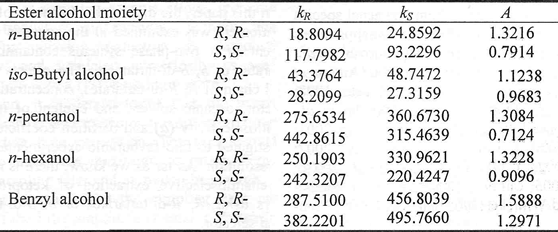
D. The effect of different alkyl chains of R, R-di-tartarates
Extraction of racemic mixtures is dependent on the difference in stability of the two diastereomeric complexes which are formed by chiral selector with the two enantiomer, So chiral extraction performance is related to the structure of chiral selector. It is very necessary to study the influence of different alkyl chains of R, R-di-tartarates or S, S-di-tartarates on k and α of both ketoprofen enantiomer, with the chiral selector concentration of 0.2 mol/L, 0.5 g/L ketoprofen, 50 % methanol and 1, 2-chloroethane as organic solvent. It is seen from Table 1 that kS is always bigger than kR, that is to say, the complexes of R, R-di-tartarates with ketoprofen S are more stable than that of R, R-di-tartarates with ketoprofen R, while the S, S-di-tartarates possess reversed characteristics. The partition coefficients and enantiose-lectivity increase with the addition of length of alkyl chain of R, R-di-tartarates or S, S-di-tartarates. Relativing to the other chiral selectors, R, R-di-benzyltartarate or S, S-di-benzyltartarate has better performances of chiral extraction. Because the aromatic rings of R, R-di-benzyltartrate or S, S-di-benzyltartrate might allow for a more stereospecific and stronger interaction with aromatic ring of ketoprofen, which increases the stabilization of complexes of chiral selector with ketoprofen molecules. But then, in the following study, we used R, R-di-butyltartarate (DBT) as chiral selector in view of R, R-di-benzyltartrate's high cost.
Table. 1 The influence of different alkyl chains of chiral selector on chiral extraction
E. The effect of organic solvent
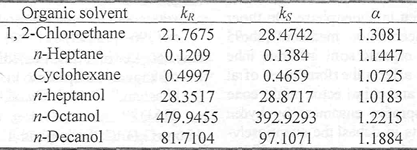
Chiral extraction performance is not only related to the structure of chiral selector, but also to the properties of organic solvents. So it is very important to investigate the influence of different organic solvent on k and α of ketoprofen enantiomers, with the R, R-DBT concentration of 0.2 mol/L, ketoprofen of 0.5 g/L and 50 % methanol. From Table 2, we can see that the extraction performance for the three kinds of organic solvents is different, for example, 1, 2-dichloroethane > heptane > cyclohexane, which might be related with the polarity and interacts of different organic solvent with solute. The partition coefficients and enantioselectivity generally increase with the addition of length of alkyl chain of alcohol, n-octanol owns perfect extraction performances.
Table 2 The influence of organic solvent on chiral extraction
F. The effect of concentration of chiral selector
The effect of the concentration R, R-DBT on k and α were investigated under the following conditions: 0. 5 g/L ketoprofen, 50 % methanol and 1, 2-chloroethane as organic solvent. It is found from Fig. 1 that the performances of chiral extraction increase with the increase of concentration of R, R-DBT. Because R, R-DBT forms complexes with the ketoprofen enantiomers, the increase of concentration of DBT facilitates the production of complexes. However, the performances of chiral extraction only increase slightly when the R, R-DBT concentration is above 0.2 mol/L. So the concentration of R, R-DBT was better about 0.2 mol/L.
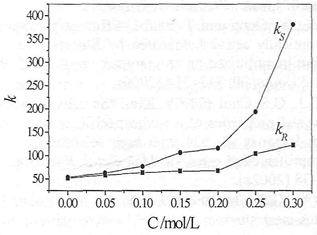
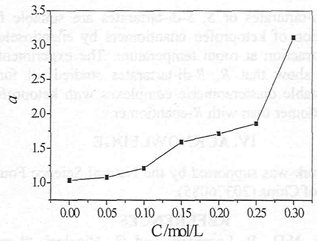
Fig. 1 Influence of the concentration of R, R-DBT on chiral extraction
G. The effect of content of methanol
The effect of the content of methanol on k and α were investigated under the following conditions: 0. 5 g/L ketoprofen, 0.2 mol/L R, R-DBT and 1, 2-chloroethane as organic solvent. We can see from Fig. 2, the performances of chiral extraction decrease with the increase of methanol content. The changes in enantioselectivity maybe due to two different phenomena. First of all, the lower concentration of methanol influences the solubility of ketoprofen in aqueous phase. On the other hand, the higher concentration of methanol (above 95 %) might cause part organic solvent infiltrate into the aqueous phase which affects the performances of chiral extraction. Methanol and chiral selector might compete in actions with ketoprofen enantiomers by hydrogen bonding, which results in debasing the enantioselectivity. So the content of ethanol was better about 50 %.
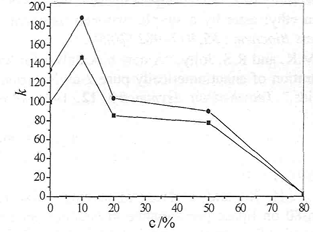
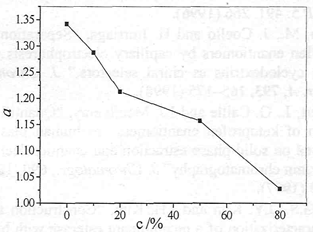
Fig. 2 Influence of the content of methanol on chiral extraction
III. CONCLUSIONS
R, R-di-tartarates or S, S-di-tartarates are suitable for separation of ketoprofen enantiomers by enantioselec-tive extraction at room temperature. The experimental results show that R, R-di-tartarates studied all form more stable diastereomeric complexes with ketoprofen S-enantiomer than with R-enantiomer.
IV. ACKNOWLEDGE
This work was supported by the Natural Science Foundation of China (20376085).
REFERENCES
1. Antona, N.D., P. Lombardi and G. Nicolosi, "Large scale preparation of enantiopure (S)-ketoprofen by biocatalysed kinetic resolution", Process Biochem., 38, 373-377 (2002). [ Links ]
2. Babin, J.E. and G.T. Whiteker, "Asymmetric syntheses", USP 5. 491. 266 (1996). [ Links ]
3. Blanco, M., J. Coello and H. Iturriaga, "Separation of profen enantiomers by capillary electrophoresis using cyclodextrins as chiral selectors", J. Chromatogr. A, 793, 165-175 (1998). [ Links ]
4. Boisvert, J., G. Caille and I.J. Mcgilveray, "Quantification of ketoprofen enantiomers om human plasma based on solid-phase estraction and enantioselective column chromatography" J. Chromatogr., 690, 189-193 (1997). [ Links ]
5. Choi, G.S., J.Y. Kim and J.H. Kim, "Construction and characterization of a recombinant esterase with high activity and enantioselectivity to (S)-ketoprofen ethyl ester", Protein Expr. Purif., 29, 85-93 (2003). [ Links ]
6. Crescenzo, G.D., A. Ducret and M. Trani, "Enantioselective esterification of racemic ketoprofen in non-aqueous solvent under reduced pressure", J. Mol. Catal. B Enzym., 9, 49-56 (2000). [ Links ]
7. Davies, N.M., "Methods of analysis of chiral non-steroidal anti-inflammatory drugs", J. Chromatogr. B, 691, 229-261 (1997). [ Links ]
8. Eikeren, P.V., F.X. McConville and J.L. Lopez, "Process for resolving chiral acids with l-aminoindan-2-ols", USP 5. 677. 469 (1997). [ Links ]
9. Fadel, A., "Optically active arylpropionic acids from the stereoselective alkylation of chiral imide enolates", Synlett., 47-50 (1992). [ Links ]
10. Heldin, E., K.J. Lindner and C. Petterson, "Tartaric acid derivatives as chiral selectors in liquid chromatography", Chromatographia, 32, 407-416 (1991). [ Links ]
11. Jaeger, K.E. and M.T. Reetz, "Microbial lipases form versatile tools for biotechnology", Trends Biotechnol., 16, 396-403 (1998). [ Links ]
12. Jin, J.N., S.H. Lee and S.B. Lee, "Enzymatic production of enantiopure ketoprofen in a solvent-free two-phase system", J. Mol. Catal. B Enzym., 26, 209-216 (2003). [ Links ]
13. Jérôme, L., G.G. Catherine and T.H. Sonya, "Efficient enantioselective extraction tris (diimine) ruthenium (ii) complex by chiral, lipophilic trisphat anions", Angew. Chem., 39, 3695-3697 (2000). [ Links ]
14. Kato, K., Y. Gong and T. Salto, "Efficient preparation of optically active ketoprofen by Mucor- javanicus lipase immobilized on an inorganic support", J. Biosci. Bioeng., 90, 332-334 (2000). [ Links ]
15. Kim, G.J., G.S. Choi and J.Y. Kim, "Screening, production and properties of a stereospecific esterase from Pseudomonas sp. S34 with high selectivity to (S)-ketoprofen ethyl ester", J. Mol. Catal. B Enzym., 17, 29-38 (2002a). [ Links ]
16. Kim, J.Y., G.S. Choi and Y.J. Kim, "A new isolate Bacillus stearothermophilus JY144 expressing a novel esterase with high enantioselectivity to (R)-ketoprofen ethyl ester: strain selection and gene cloning", J. Mol. Catal. B Enzym., 18, 133-145 (2002b). [ Links ]
17. Kim, M.G., E.G. Lee and B.H. Chung, "Improved enantioselectivity of Candida rugosa lipase towards ketoprofen ethyl ester by a simple two-step treatment", Process Biochem., 35, 977-982 (2000). [ Links ]
18. Kumar, M.K. and R.S. Jolly, "A new biocatalyst for the preparation of enantiomerically pure 2-arylpropano-ic acids", Tetrahedron Asymmetry, 12, 1431-1434 (2001). [ Links ]
19. Laue, C., G. Schroder and D. Arlt, "Intermediates and their use in the preparation of S-ketoprofen", USP 5. 362. 907 (1994). [ Links ]
20. Liu Y.Y., J.H. Xu and Y. Hu, "Enhancing effect of tween-80 on lipase performance in enantioselective hydrolysis of ketoprofen ester", J. Mol. Catal. B Enzym., 10, 523-529 (2000). [ Links ]
21. Liu Y.Y., J.H. Xu and H.Y. Wu, "Integration of purification with immobilization of Candida rugosa lipase for kinetic resolution of racemic ketoprofen", J. Biotechnol., 110, 209-217 (2004). [ Links ]
22. Lukas, H., O. Schuster and G. Rau, "Process to separate mixtures of enantiomeric arylpropionic acids", USP 4. 983. 765 (1991). [ Links ]
23. Madhav, M.V. and C.B. Ching, "Study on the enzymatic hydrolysis of racemic methyl ibuprofen ester", J. Chem. Technol. Biotechnol., 76, 941-998 (2001). [ Links ]
24. Manimaran, T. and A. A. Potter, "Resolution of ketoprofen", USP 5. 162. 576 (1992). [ Links ]
25. Park, H.J., W.J. Choi and E.C. Huh, "Production of optically active ketoprofen by direct enzymatic esterification", J. Biosci. Bioeng., 87, 545-557 (1999). [ Links ]
26. Ramminger, C., D. Zim and V.R. Lando, "Transition-metal catalyzed synthesis of ketoprofen", J. Braz. Chem. Soc., 11, 105-111 (2000). [ Links ]
27. Rossi, R.F., D.L. Heefher and C.M. Zepp, "Enantiose-lective production of chiral carboxylic acids", USP 5. 273. 895 (1993). [ Links ]
28. Shen, D., J.H. Xu and H.Y. Wu, "Significantly improved esterase activity of Trichosporon brassicae cells for ketoprofen resolutionby 2-propanol treatment", J. Mol. Catal. B Enzym., 18, 219-224 (2002). [ Links ]
29. Shimizu, I., Y. Matsumura and Y. Arai, "Process for preparing alpha-(3-benzylphenyl) propionic acid derivative", USP 4. 999. 450 (1991). [ Links ]
30. Vladimir, P., K. Mice and E. Martin, "Lipophilic tartaric acid esters as enantioselective ionophores", Angew. Chem., 28, 1147-1152 (1989). [ Links ]
31. Xi, W.W. and J.H. Xu, "Preparation of enantiopure (S)-ketoprofen by immobilized Candida rugosa lipase in packed bed reactor", Process Biochem., 40, 2161-2166 (2005). [ Links ]
32. Yoneyoshi, Y., J. Kudo and T. Nishioka, "Optically active secondary amine compound, process for producing optically active secondary amine compound and process for producing optically active carboxylic acid by using said compound", USP 5. 510. 519 (1996). [ Links ]
Received: April 28, 2006.
Accepted: May 31, 2006.
Recommended by Subject Editor R. Gomez.














