Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Latin American applied research
Print version ISSN 0327-0793
Lat. Am. appl. res. vol.36 no.4 Bahía Blanca Oct./Dec. 2006
New material as support for nickel boride catalyst
D. Acosta*, J. Martinez, C. Carrera, E. Erdmann, E. Gonzo and H. Destéfanis
Consejo de Investigaciones de la UNSa-CIUNSa- Instituto de Investigaciones para la Industria Química- INIQUI-CONICET, Facultad de Ingeniería-Universidad Nacional de Salta- Dirección: Buenos Aires 177-4400-Salta-ARGENTINA.
* acostad@unsa.edu.ar
Abstract — The main objective of this work is to study the feasibility of new materials to be used as support for boron-nickel catalysts. Potential support materials such as: silica gel, alumina, hydrothermal modified Perlites and zeolite 4A, were characterized by BET, TPR and DRX. After the addition of Ni and B, their catalytic activity evaluated with the nitrobenzene hydrogenation model reaction. The influence of operational parameters during the impregnation process such as order of reactants, speed of agitation, time of aging and it influences from the previous thermal treatment were evaluated. The results show that the adequate materials to be used as catalysts supports are the commercial silica and Rehydroxilated Perlite. It is due to the existence of superficial OH groups, which allow the anchorage of the nickel boride catalyst.
Keywords — Supported Catalyst-Nickel Boride-Perlite.
I. INTRODUCTION
Amorphous alloys have properties that are of interest in catalysis: (a) the presence of a large number of surface coordinating unsaturated sites, (b) the lack of crystal defects, and (c) the isotropic, single phase nature of the materials (Smith et al., 1980; Molnar et al., 1988;). So that one of the constant searches in catalysis is to achieve surface uniformity in order to an industrial development. Since 1980, the amorphous metal-metalloid alloys received special attention for its excellent catalytic selectivity and activity properties. Two techniques are the most frequently employed in preparing amorphous alloy catalysts. One is a technique of fast blended; however the specific surface area of these materials is very low (0.1-1 m2/g). The other one involves the reduction of transition metals for action of BH4 - and/or H2PO2 - that produce ultra fine amorphous alloy particles (Shibata et al., 1985; Yoshida et al., 1986). Although the active surface area increases by one or two order of magnitude due to their nanosized dimensions, their low thermal stability makes their structure very unstable at high temperature (Linderoth and Morup, 1991). However, when through metal-support interactions, it is possible to block the diffusion pathway of the atoms, which is the pre-requisite for crystallization.
Among the materials commonly used as catalytic supports are the silica and the alumina. In our region, (Salta, in the Northwest of Argentina) exists abundance of natural silicoaluminate, which could be used as excellent systems for supports because of its chemical inertia and the feasibility for controlling the surface by means of appropriate transformations.
The first step of the study was focused in the selection of the capable materials to be used as support, their characterization and adaptation to the reacting system. Commercial supports as silica gel, alumina, and modified materials obtained from expanded Perlite were used. Modification of the Perlite was carried out through hydrothermal treatments. To obtain Rehydroxilated Perlite the hydrothermal treatment was carried out in vapor phase at different temperatures (Abalos et al., 2003). The process to obtain zeolite from expanded Perlite was carried out in liquid phase with alkaline solutions of variable concentrations of NaCl (Bottale et al., 1996). In those papers the best conditions to obtain Rehydroxilated Perlite and also Zeolite 4A are discussed. Also, the physical-chemical characterization was analyzed using BET, FTIR and DRX. The main objective of this work is to study the feasibility to support catalysts of the boron-nickel type, and to evaluate its catalytic aptitude for a potential use in industrial processes.
The preparation of the catalysts was done following a previously developed procedure (Destéfanis et al., 1992) in order to obtain the same active specie that in the case of the massive catalyst. The influence of the operative parameters during the process of impregnation as the order of reactants, speed of agitation, time of aging and the influence of thermal pretreatment on the reaction rate, were evaluated.
II. METHODS
Supports preparation: The materials used as supports are classified in three groups:
Rehydroxilated Perlite: Perlite is volcanic glasses that can be expanded when they are exposed to high temperatures. Hydrate water is eliminated during this expansion process. The obtained material is denominated expanded Perlite with characteristics such as low chemical reactivity, high porosity and mechanical resistance. Starting with expanded Perlite is possible to obtain Rehydroxilated Perlite (Abalos et al, 2003) through a technique that consists in exposing the solid to water vapor at high pressure in a PARR Reactor (Model 4652) at different times of treatment.
Zeolite: The zeolite was obtained from expanded Perlite, treating them with alkaline solutions of NaCl, with variable concentrations of Al varying the time of treatment (Bottale et al, 1996).
Commercial Supports: Silica Gel (Davison Chemical) grade 923, mesh 100 - 200. Alumina (ALCO) in pellets.
Catalyst Preparation: Exactly 1 g of different supports was immersed in a desired amount of aqueous solution of sodium borohydride (NaBH4, 2.0M and NaOH 0.2M) at 293K for 0.5 h. After excess solution was removed by filtration, the impregnated support was transferred to a beaker in an ice-water bath and a nickel acetate aqueous solution (Ni(CH3CO2)2.4 H2O, 1.0M) was added drop wise with gentle stirring. When no more bubbles were generated, the sample was washed with 99.9% ethanol for its characterization or catalytic activity test.
Characterization: Studies of X-Ray Diffraction (DRX) in a Rigaku Denki D-max-IIC with Ni filtered Cu Kα radiation was carried out. Nickel and boron content in catalysts were analyzed by Atomic Absorption Spectrometer. The surface area was measured by BET method with N2 at 77 oK on a Micromeritic Flor Sorb Model 2 - 2300. The degree of surface corrosion in the Rehydroxilated Perlite was followed by Infrared (FTIR) Brucker using diluted samples in KBr and pressed at 4 tn/cm2.
Activity catalytic test
The hydrogenation reaction was carried out in a Parr Reactor Model 3912 containing 1.0 g of catalyst in tetrahydrofurane (THF) and 26 ml of Nitrobenzene in methanol as a solvent. The autoclave was purged with H2 four times to exclude air. When the desired reaction temperature was achieved, the H2 pressure was adjusted to 3.7 at. In order to study the intraparticle diffusion effect, we followed the procedure proposed by Madon and Boudart (1982) by varying the amounts of catalyst used in the reaction system or by changing the loading of the catalyst. Under these conditions the hydrogenation reactions is carried out in kinetically controlled region (Destéfanis et al., 1989).
II. RESULTS AND DISCUSSION
Rehydroxilated Perlite
The hydrothermal treatment of expanded Perlite leads to the formation of free silanol groups on the surface (Abalos et al., 2003). The concentration of these groups is strongly dependent of temperature and time of treatment. These parameters were determined using Perlite >300 mesh. The hydrothermal treatment results in a surface modification, which affects the support surface area. It could be inferred from the increasing specific surface area with time as shown in (Table 1) and by the OH stretching band in FTIR (Figure 1). However after 90 hours of treatment the BET area decays.
Table 1: Surface and adhesion of material used as Nickel Boride support
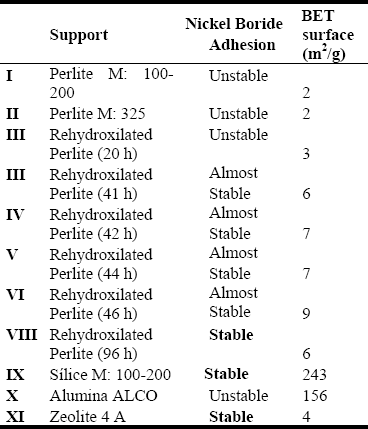

Figure 1: FTIR of Rehydroxilated Perlite vs. time
(a) 46 hr. (b) 43,5 hr. (c) 42 hr. (d) 96 hr. (e) Expanded Perlite
Fig 1 shows the characteristic vibration O-H at 3700 cm-1 corresponds to the frequency νSiO-H and it is confirmed with O-H deformation at the 870 cm-1. For increasing time of hydrothermal treatment, the OH bands show a maximum at 46 h. After that, a degradation process can be assumed due to a decreasing in the BET area and a starting crystallization process as can be seen by X-ray. In spite of those results, hydrothermal treatment at 96 h shows OH stretching vibration by FTIR. From these results it can be assumed that hydrothermal treatment modifies the surface for superficial corrosion creating free places of SiOH during the time of treatment. For SiO2 as support FTIR made in vacuum shows that sylanols group (SiOH) is present over the surface by strong absorption band at 3741 cm-1, which is assigned to free νSiO-H (Haukka, 1993) next to a broad band at 3500-3680 cm-1 due to hydrogen bridge bonds. Zeolite and Alumina do not have free OH group.
The X-Ray of the starting materials (expanded Perlite) and those obtained by rehydroxilation process at different times, show that all samples remain amorphous confirming that the thermal treatment does not induce a significant crystallization growth.
Zeolite Perlite: Zeolite 4A
The hydrothermal treatment with NaCl/Al in an appropriate relationship of Si/Al modifies the material to obtain Zeolite 4A (Bottale et al., 1996). The selection of this type of zeolite was made because its holes are proper to support the catalyst in a later stage. Figure 2 shows DRX of all the supports used. The expanded Perlite (starting material for modification to Rehydroxilated or zeolitization) is initially amorphous. The hydrothermal treatment do not change the crystalline structure of the rehydroxilated Perlite and remains amorphous, while the zeolitization process leads to a crystalline material. It can be probe by comparing with the diffraction pattern for pure Zeolite 4A which corresponds to a structure of Hydrated Linde A (Na96Al96Si96O384 (H2O)216).
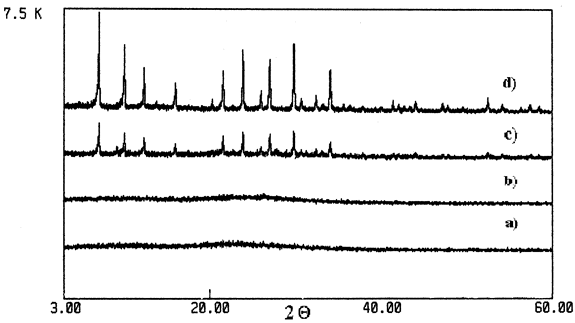
Figure 2: X-Ray Diffraction of different supports
a) Expanded Perlite, b) Rehydroxilated Perlite 43 h., c) Zeolite 4A from Perlite, d) Zeolite 4A Pure
All these materials were used as supports to prepare nickel boride catalysts with the same protocol using 1g of support. The process of impregnation was carried out with cold alkaline solutions to avoid the decomposition of sodium borohydride and to preserve its reducing capacity. Tests altering the impregnation sequence (solution of sodium borohydride and then methanol solution of nickel acetate) were done. The results confirm that the better sequence is when the impregnation of sodium borohydride is used first. This election was based in the fact that the resulting material has higher specific surface area and a better catalyst adhesion to the support. Since nickel boride is a black precipitate of very small particle size, it could pass through the pores of the filter if the nickel boride is not properly anchored to the surface of the support. The resulting black solution shows the null surface adhesion. This last condition is indicated on Table 1 as "unstable".
Activity Test
The nitrobenzene hydrogenation rate is a first order in hydrogen pressure as can be seen in Fig 3. The slope of the straight line is the first order rate constant k (assuming constant temperature and catalyst concentration).
 | (1) |
Equation (1) can be modified by the Henry law CH2 = PH2 /k (2) and solved with initial conditions, PH2= PH2o at t=o (Destéfanis et al, 1989)
 | (3) |

Figure 3: Nitrobenzene Hydrogenation over BNi/SiO2 To= 328oK, PH2= 3.6 atm
In order to compare activities of different supported catalyst and bulk nickel boride in nitrobenzene hydrogenation, several tests were carried out. With bulk nickel boride catalyst the kinetic behavior was the same as that mentioned above for supported catalyst. The specific rate constant per gram of Ni was almost equal to nickel boride over SiO2 than bulk nickel boride (Table 2).
Table 2: Specific Rate Constant Value for Nitrobenzene Hydrogenation

Table 3 shows the constant rate for nitrobenzene hydrogenation with rehydroxilated Perlite treated at different times. The values of kinetic constant were obtained doing each experiment by triplicate (standard deviation of 5 x10-7). The hydrogenation rate for the rehydroxilated Perlite is the same at different treatment times, such as 20 h, 40, 96 h. This is consistent with the degree of hydroxylation after 20 h, where the quantity of the hydroxyl groups over the surface reach a plateau (Abalos et. al, 2003). A slight increase in the rate of hydrogenation is observed because the silica material presents higher BET area. Nevertheless, the results show that the kind of boride formed over the surface is the same using silica as a support than using a rehydroxilated Perlite. Activity test of nitrobenzene hydrogenation carried out using Zeolite as support shows a very low reaction rate. Due to the fact that zeolite do not have free OH group on the surface the results confirms that the nickel boride obtained probably anchorage to the support through the free OH groups over the surface.
Table 3: Rate Constant Value over Different Support

III. CONCLUSIONS
According to these results potentials supports for catalysts, are commercial Silica and rehydroxilated Perlite treated between 20-45h. FTIR analysis points out that this property could be associated to the existence of superficial OH groups that allow the anchorage of the nickel boride. Supported and non-supported catalyst shows the same kinetic behavior in the nitrobenzene hydrogenation.
REFERENCES
1. Abalos, R.; Erdmann, E. y Destéfanis, H.A. "Surface modifications of volcanic glasses (perlites) by water vapor". Latin American Applied Research, 33, 59-62, (2003). [ Links ]
2. Bottale, M.G.; Acosta, D.E.; Destéfanis, H.A. "Síntesis de zeolita por tratamiento hidrotérmico de perlita. Efecto de la concentración de cloruro de sodio". Información Tecnológica, Revista Internacional Arbitrada. Editorial del Norte Ltda. Chile. Vol 7, No6, (1996). [ Links ]
3. Destéfanis H. A., D.E. Acosta, E.E. Gonzo Hidrogenación de Nitrobenceno con Catalizadores de B3 -Ni4. Actividad Catalítica y Cinética"., VI Jornadas Argentinas de Catálisis, Huerta Grande, -Córdoba: 19 al 22 de Septiembre, pg 3-4, (1989). [ Links ]
4. Destéfanis, H.; Acosta, D.; Gonzo,E. "Preparation of Metal Borides in an Aprotic System", Catal. Today, 15, 555-564, (1992). [ Links ]
5. Haukka, S., "Characterization of Surface Species Generated in Atomic Layer Epitaxy on Silica", Doctoral Thesis, Helsinki , Sweden, (1993) [ Links ]
6. Hui Li, Hexing Li, Jing- Fa Deng. "Hidrogenación de Glucosa sobre el catalizador Ni-B/SiO2 amorfo y el efecto de promoción de metales dopantes", Catalysis Today, 74, 53-63, (2002). [ Links ]
7. Linderoth, S. Morup, J. Appl. Phys. 69, 5256 (1991). [ Links ]
8. Molnar A., G.V. Smith, M. Bartok, in: D.D. Eley, H. Pines, P.B. Weisz (Eds.), Advances in Catalysis, Vol. 36, Academic Press, New York, 329, (1988). [ Links ]
9. R.J. Madon, M. Boudart, Ind. Eng. Chem. Fundam. 21 (1982) 438. [ Links ]
10. Smith G.V., W.E. Brower, M.S. Matyjaszczyk, T. Pettit, 7th Proc. Int. Cong. Catal. Tokio, 355, (1980). [ Links ]
11. Shibata S., Y. Ohbayashi, N. Kawata, T. Masumoto, K. Aoki, J. Catal. 96, 296, (1985). [ Links ]
12. Yoshida S., H. Yamashita, T. Funabiki, T. Yonezawa, J. Chem. Soc. Chem. Commun. 964, (1986). [ Links ]
Received: December 31, 2005.
Accepted for publication: June 20, 2006.
Recommended by Subject Editor J. M. Pinto.














