Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Latin American applied research
versão impressa ISSN 0327-0793
Lat. Am. appl. res. v.37 n.2 Bahía Blanca abr. 2007
A new process to obtain palladium as metal powders from salts: thermodynamic an kinetic study
M. Villicaña1, M. G. Garnica-Romo2, J. F. Pérez-Robles3 and J. A. Cortes1
1 Facultad de Ingeniería Química, Universidad Michoacana de San Nicolás de Hidalgo, Morelia, Mich., México
2 Facultad de Ingeniería Mecánica, Universidad Michoacana de San Nicolás de Hidalgo, Morelia, Mich. México.
gromar05@yahoo.com.mx
3 CINVESTAV-IPN. Unidad Querétaro. Querétaro, Qro., México
Abstract — In this work, a new process to obtain palladium powders from their salts by using a cementation process (in aqueous solution) through an iron electrode and a catalyst agent was studied. The process gives results that in practice can also be applied to obtain bulk metal from secondary sources. Nano and micrometric particles could be obtained from this new process. The physicochemical and morphological characteristics of these powders are heavily dependent on the method used for their production. For the Palladium test and PdCl2 solution 0.093 M, a minimum quantity of catalyst agent was added to accelerate the reaction. In the palladium powders only small round particles were obtained, which can be easily suspended in solution. For the reaction, there is no considerable temperature dependence. Thus, the process can be carried out at room temperature resulting in considerable energy saving.
Keywords — Palladium Metal Powder. Nanometric Particles. "Black" Palladium Powder.
I. INTRODUCTION
Palladium is obtained through complexing agents. In copper minerals there are low quantities of metals from the platinum group, mainly platinum and palladium, which are recoverable during electrolytic refining of copper from various sources or by scrap precious metals refining. The filtered extract from platinum recovery contains palladium chloride. The latter is treated with hot ammonium hydroxide, forming, in solution, a complex called tethramminopalladium chloride (II) [Pd(NH3)4]Cl2..H2O]. Adding HCl to the latter precipitates the yellow, insoluble compound dichloride-diamminopalladium PdCl2(NH3)2, which is rapidly soluble in cold and diluted ammonia. This series of reactions are very efficient for producing high-purity salts, from which the metal is obtained by heat. Palladium is obtained at industry-scale, heating [Pd(NH3)4]Cl2 until white-red; leaving the metal in the form of a soft powder or a sponge-like form.
Palladium chloride precipitation in the form of dimethyl-glioxima (a selective precipitant) must be done in a slightly acidic solution to avoid contamination from nickel. Palladium chloride is an orange-yellow precipitate, formula Pd(HC4H6N2O2)2, that transforms into the metal by calcinations (Cotton and Wilkinson, 1964; Sergueiev, 1975).
Fine and uniformly sized and shaped black palladium powders have been obtained by the Polyol process from an inorganic precursor and by chemical reduction into ethylene-glycol (Bregado and Campos, 1988; Begrado, 1989; Ferrier et al., 1985; Figlarz et al., 1989). The Mono-dispersed spherical particles (average size of 0.1 micron) are produced from hydrazine (Pd(NH3)4)2+ in ethylene-glycol in the temperature range of 20o C to –9oC, required by the micro-electronic industry (Pérez, 1995). The physical-chemical and morphological properties of these powders depend largely on the process used to obtain them (Contreras, 1960; Hedley and Tabachnick, 1958). The properties of these materials are generally obtainable in ultra-dispersed micrometric or submicron particle size materials. It is worth noting that the properties of these materials turn out to be very different from massive materials obtained by processes such as smelting. This is due to an increase in the solid surface, as a consequence of a high-dispersion state when in powder form (Matijevic and Hsu, 1985). There are some properties whose increases will be considerable, such as: specific surface, reactivity before and after the synthesizing process and even the system's free energy which is formed.
This new wet process to obtain or recover metallic particles is really a catalyzed cementation process. (Pérez et al., 2001). In this process, the chemical precipitation of a metal is performed, generally from an aqueous solution of its salts, by a more electro-positive metal electrode on a shorter time-span; these potentials cannot be measured directly even though their relative values can be determined with respect to a half-cell of reference. The most frequently used is the Standard Hydrogen Electrode (SHE). Metallic salts are dissolved in water and a catalytic agent is added, which is a solid salt or a liquid. An iron electrode is then immersed in the solution. The precipitation of the metal is immediately observed on the electrode-solution interphase and the spongy precipitate is recovered by a mechanical scraper. The obtained precipitate settles, is washed abundantly with water, and is dried and weighed to quantify the reaction's yield. The Thermodynamic, Kinetic and Activation Energy parameters are then determined. The process can be used as an intermediate stage to obtain metals en-masse from secondary sources.
The physical, chemical and morphological characteristics of these powders mainly depend upon the concentration and the catalytic agent being used. The global process involves the formation and recovery of metallic powders from the metal salts and by-product recovery. Metals more electro-positive than iron precipitate according to the general reaction characteristic of this process (Eq. 1):
3 Mex+ + Feo → 3Meo + xFe3+ (1)
II. METHODS
A 40 ml palladium chloride solution was prepared (II) PdCl2 0.09305 N. A minimum fluoride ion quantity was added to both tests as a catalyst to accelerate the reaction, which is heavily dependant on the electrode's exposed surface. The catalyst addition was 0.1188 mol/l of fluoride ion; but this can be lowered if a smaller particle size is required. The tests were conducted on a plastic precipitation glass; the electrode was installed on a mechanical stirrer (50 y 30 rpm), and was immersed in the solution. In this phase, metallic particles start being generated and removed from the electrode's surface. The ion's conversion into metal is evaluated upon stopping the reaction by removing the electrode from the solution; the precipitate was abundantly rinsed with water to remove the catalyst and the iron ion. The powders were dried at 100o C and feature DRX, SEM and LS. Total reaction time was approximately 10-12 min.
III. RESULTS AND DISCUSSION
The process is based on a modified silver cementation process applied to palladium ions with an iron electrode. The dissolution (corroding) of the iron electrode is accelerated by the presence of an oxidizing agent: the fluoride ion.
The metallic powders were precipitated in acid solutions at pH from 3.8 to 5.5. The groups of reactions that were being considered for metal precipitation are shown in Table 1, values are given on Kcal. Table 2 shows the results of the reactions described in Table 1, the values of the reactions obtained for metallic Palladium, its ions or oxide (PdO). Values on the enthalpy (ΔH) change are negative. These reactions, which dissipate some heat, are exothermic, except Reaction (2). Negative values for Gibbs' Free Energy are significant for spontaneous reactions, which mean a high tendency to produce metallic powder. In practice, the reaction has a slow reaction rate and is accelerated by adding an extremely oxidant catalytic agent (the fluoride ion) in small concentrations. Values in equilibrium constant (Keq) are high, making the reaction practically irreversible in some cases.
Table 1. Metal reactions being considered.

Table 2. Thermodynamic values of the different Chemical reactions.

A. Metallic powder precipitation mechanism
Tables 1 and 2 show the different chemical reactions and their respective physicochemical values, which are involved in the process. From Table 1's reactions, taking note of its dependence upon (E) potential, pH or both, Pourbaix's (Allens and Larry, 1967; Garrels and Christ, 1965) diagrams can be constructed assuming an ion concentration in the equilibrium of 1x10-6. This Palladium diagram was constructed in the presence of iron and fluoride ions (Fig. 1). In the conditions in which the metal was obtained, only Iron (III) was found in the working region.

Fig. 1. Eh-pH Diagram showing palladium and iron species and water stability region (dotted line) as a pH function.
Analyzing Pourbaix's Palladium diagram (Fig. 3), and noting that Eq. (6) of PdO and Eq (7) from Pd2+ have a positive (ΔG) and a very small equilibrium constant value (Keq), conversion from Pd2+ to PdO is at a very low pH (pH<1.0) and at very high potentials (E> 0.5 to 2.0). Even though the solution's initial pH is 7.0, this value can be lowered considerably by the presence of iron ions in the electrode-solution interphase. Therefore, it is possible to reach equilibrium at very low pH levels and still be within the water stability line.
From Pourbaix's Diagram for Palladium, there are two alternatives to metal production: The Pd2+/Pdo and Pd2+/PdO and later into PdO/Pdo conversion. For the latter there is a problem: It is not possible to transform PdO/Pdo. Perhaps it is because of its Kinetics or its transformation potential being unreachable, Eh, and PdO appears as a byproduct. Also, if there is not an estequiometric amount of electrons, it can produce a significant amount of palladium oxide, which appears as a reddish dust mixed with the black palladium powder, making the solution a red-brown color.
Figure 2 shows how the electrode interacts during the reaction. If a high purity metallic powder is desired, the iron electrode surface must be activated when immersed into a low concentration HF solution (2.0% or less).

Fig. 2. Solution-electrode interphase. This shows the production process for metallic powders: a) Low quantity Iron oxide (MO) interphase within the pores (black dots near the interface) which produced a metal and corresponding oxide mix. b) Non-iron oxide interphase, which only produces metal.
From Table 1 (1) reaction, it can be observed that in order to produce metallic palladium powder, the equilibrium constant is defined by the proportion Keq = [ (Fe2+)2 / (Pd2+)3 ]. For this reaction, the equilibrium constant is 1.725X1094. Assuming that the palladium ion concentration (Pd2+) is very low at equilibrium, 1X10-6 for example, and iron ion concentration (Fe3+) is very high, this means that iron ion concentration won't be a barrier to the reaction taking place until total metal precipitation is achieved.
B. Catalytic agent's effect in metallic powders precipitation.
In Fig. 5, the reaction of the fluoride ion with the iron electrode is shown increasing oxidizing reaction while reducing the interphase pH at the same time, which is attributable to the presence of dissolved iron ion. Initial reaction stages take place very quickly, with the formation of intermediate ferrous ions (II), as well as ferric ions (III) within the aqueous solution surrounding the electrode. Fluoride ions have access to the electrode surface, so reactions (8) and (9), already explained in Table 1, take place.
From the Fluoride ion attack, two compounds can be formed: FeF2 y FeF3, but ferric fluoride FeF3 is the species to subsequently attack the electrode, due to FeF3 having the higher solubility constant value (0.091 parts in 100 water parts) (Lang's, 1989), while FeF2 has an equilibrium constant Keq=2.36x10-6 (CRC Handbook, 1970) and Gibbs' free energy for FeF3 is at a much higher value than that of FeF2. This suggests that FeF3 is responsible for generating the excess electrons for reducing metallic ions into metal in the solution. Also, it dissolves and produces the necessary fluoride ion for the new electrode corrosion, forming a new amount of FeF3. This process generates the necessary electrons for metallic ion reduction into metallic powder.
C. Potential (E) Calculus for each electrochemical cell
If we start from the fundamental relation between F.E.M and free energy, the following equations are determined:
ΔG = -n F E (2)
Iron oxide Interphase
ΔH = ΔG + T ΔS (3)
Differentiating the first equation with respect to temperature and keeping pressure constant, while equating the resulting Eq. (4), we have:
ΔS = n F ( ΔE / ΔT )P (4)
To calculate Entropy change, the total reaction potential values are graphed against the temperature values used. The skew of this straight line is obtained from the entropy change in the following Eq. (5):
(ΔS / ΔT )P = m (5)
Entropy obtained value is: ΔS = 25.3227x10-3 Kcal/K
With thermodynamic equilibrium constant values (K), we can calculate the total reaction potential (E) through Eq. (6):
E = E0 + (RT / n F ) ln Q, (6)
where: E=Total reaction Potential, E0=Standard Potential, n=number of e- and OH- charges that participate in the reaction, Q=Equilibrium relation for each reaction, R=Gases constant, T=Kelvin scale temperature.
Standard Potential value and Middle cell equations are:
Pd2+ + 2e- → Pd0 0.9510 E0 (V) (7)
Fe0 → Fe3+ + 3 e- 0.0370 E0 (V) (8)
The different total Potentials of the temperature cells (E) and the equations to produce metallic powders are shown in Table 3. We can see that the minimum potential to obtain Palladium is 0.88989 V at 298oK.
3Pd2+ + 2Fe0 → 3Pd0 + 2Fe3+ 0.8898 E0 (V) (9)
Table 3. Total cell reactions and obtained Potential values.

D. Kinetics of the new process
Palladium ion conversion data in the presence of the catalyst agent were obtained at 4 different temperatures, as shown in Fig. 3. It is necessary to comment on the fluoride ion minimum concentration level in order to achieve reaction at a high proportion. This is because reaction depends completely upon corrosion of the iron electrode. In this case, the maximum conversion level (99.9%) was obtained at all temperatures, but at 298oK this conversion took place at 12 minutes. This is acceptable on an industry scale, due to the huge volume of runoff water used to recover, in this case, metallic palladium from a secondary source, or to obtain this metal from a primary source. The use of services (energy) to carry out the reaction at 50oC (323oK) saves 4 minutes of the total reaction time and is not advantageous. The tests were conducted at a very low palladium concentration (0.0905N) due to the salt's high price. The total electrode area used in the tests was 11.00 cm2, and the total solution volume was 40 ml.

Fig. 3. Graphic depiction of palladium ion conversion into metallic palladium.
As Fig. 4 shows, the effect of temperature on the reaction kinetics is significant for scientific results, but not for processes of recovering heavy metals. Therefore, the reaction can be performed at room temperature. These are considered to be first order, homogeneous, spontaneous reactions, according to Mass-Action Law and are expressed by the following equations Eqs. (10-12) (Habashi, 1970):
r = dCA / dt r = K CA , (10)
where: CA is metallic ion concentration over a t time.

Fig. 4. Palladium control mechanism at three different oC scale temperatures
Deriving the prior equation, integrating between limits, we obtain:
dCA / dt = K CA (11)
ln ( CAo / CA ) = Ke t, (12)
where Ke is the reaction speed specific constant.
E. Determining Reaction order.
Figure 5 shows the experimental data ln(CAo/CA) vs. t, analyzed by a graphic method, which demonstrates a linear behavior for the temperatures studied, and corresponds to the proposed reaction order (first order reaction) (Logan, 2000). Using graphical methods, when ln(CAo/CA) vs. t is plotted, an origin-intercepting straight line must be obtained; this indicates that the assumed order is correct (first order reaction) as shown in Fig. 4.

Fig. 5. Graphic depiction of ln Ke vs 1/T (oK), and Palladium Activation Energy calculus.
F. The effect of temperature on reaction speed and Activation Energy (Ea).
Le Chatelier-Braun's principle establishes that temperature's effect upon reaction speed is given by the temperature level and the Activation Energy (Ea) (Metz, 1976). Temperature increase leads to a considerable increase in reaction speed, hence different reaction speed specific constant values. Arrhenius was the first to indicate that this variation is represented by
K = A e -(Ea/RT) (13)
where Ea is the Activation Energy.
A variation between Ea and temperature indicate a change upon reaction's controlling stage and a high Ea value corresponds to a more temperature variation-sensitive reaction (Levenspiel, 1987; Maron and Prutton, 1974). From Eqs. 12 and 13, the energy of the process can be evaluated. In Fig. 5, ln Ke against 1/T in oK values for obtained metallic powders are represented. The data was adjusted into a straight line and the skew of each straight line is (– Ea / R) = m. The palladium Activation Energy value (Habashi, 1970) is Ea = 6.869 Kcal/mo. This corresponds to the chemical reaction's controlled processes in the interphase. This is because metallic ion conversion into metal is directly related to the production of free electrons from the iron electrode oxidation.
G. Powder identification and characterization
Metallic powders were initially deposited on the electrode surface and were mechanically scraped from the electrode surface into the solution. Large particles precipitate slowly towards the bottom of the reaction glass. The metallic powder was recovered by decantation, and powders were water-washed to eliminate all traces of iron and the probable ferric oxide produced by the electrode. X-ray diffraction pattern and morphology in metallic powders can be observed (Fig 6 and 7). Palladium powder X-ray diffraction shows main crests at 40.118, 46.658 and 68.119 respectively. These figures show metal only, not oxides, which suggests the purity of production. Particles produced were different sizes, rounded, uniformly shaped with some clustering (Fig. 7).
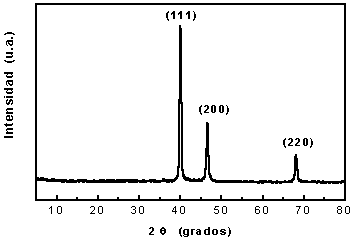
Fig. 6. X-ray of obtained Palladium powder

Fig.7. Diffraction pattern and morphology of obtained palladium powder.
Particle growth is probably due to a heterogeneous nucleation mechanism. Solid nuclei are preferentially generated on convex surfaces when the substrate radius (in this case the electrode pore) is small (*), hence, the electrode wall gaps must be big enough to allow the solid to grow on the outside. Therefore, the solid-liquid interphase radius must be larger than the critical nucleus one (r*). Even though there is a high density solid nucleus, the ion radius is smaller than that of palladium ions (0.86:1 Å). The electrode pores were small enough to allow fine grain size to diminish and a rounded grain structure of a different size could then be obtained. Produced powders were put into a dispersion process on a high energy SPEX mill in the presence of Jicama starch as a dispersion agent at different dispersion times, obtaining a stable suspension. Figures 8-9, show size distribution at different dispersion times obtained with LS. In the first sample (fine), particles have a 0.055 microns = 55 nm (Fig. 8) Gaussian distribution. Those obtained 48 hours later were larger in size.

Fig. 8. Fine Palladium powders with a 2%

Fig. 9. Thick palladium powder (upper part) weight increase for Jicama starch. Suspensions with a 2% weight increase for Jicama starch and 52 hours inside the SPEX.
Figures 8-9 show an increase in particle size by the addition of the dispersion agent; however, the dispersing concentration percentage does not change. Size increase is more due to the clustering effect as a function of dispersion time. Starch pectin chains being broken explain clustering. Most probably, the broken starch chains react with the particles' surface, making size increase due to coalescence. In the last Figure, an average 20.8-micron size is shown.
IV. CONCLUSIONS
In this study, a novel process for obtaining metallic black palladium powders from primary sources was conducted. Thermodynamic and kinetic results show that the process is technically viable and that Palladium powder acquisition speed is not dependant on temperature when using an iron electrode. The electrode can be easily found on the market or cheaply produced. The process is slightly exothermic and can be performed at room temperature or near to it. This makes it easy to escalate the process to a commercial level combined with low energy consumption costs. Activation Energy for the process is on the order of 5-10 Kcal/mol, characteristic for non temperature-sensitive reactions, thus additional energy is not required. X-ray analysis of the obtained powders shows the metal's characteristic crests; no oxides appear. Furthermore, they show a perfectly rounded circular morphology averaging 55 nanometers for the finer powders.
ACKNOWLEDGMENTS
To PROMEP of Mexico, for their financial support for this research.
REFERENCES
1. Allen, J.B. and R.F. Larry, Electrochemical Methods, Fundamentals and Application. John Wiley & Sons. New York (1967). [ Links ]
2. Begrado, J. and Y. Campos, "Modelos Teóricos de sinterización." Boletín de Información Científico del Centro de Investigaciones Metalúrgicas, 9, 30-59. (1988). [ Links ]
3. Bregado, J. "Caracterización morfológica de polvos Metálicos mediante M:E:B". II Congreso de la Federación Iberoamericana de Biología Celular y Molecular, III Congreso de la Sociedad Iberoamérica de Histología e Histoquímica, VII Congreso de la Sociedad Latinoamérica de Microscopia Electrónica. La Habana Cuba. (1989). [ Links ]
4. Contreras, C.D. "Experimentación de minerales de oro y plata por el proceso de Cianuración". Comisión de Fomento Minero, Boletín No. 8, México, D.F. (1960). [ Links ]
5. Cotton, A.F. and G. Wilkinson, Química Inorgánica Avanzada. Mexico. 1057-1061. (1964). [ Links ]
6. CRC. Handbook of Chemestry and Physics. 67th Ed., B-207. (1970). [ Links ]
7. Ferrier, G.G., A.R. Berzins and N.M. Davey, "The Production of Palladium Powders for Electronic Applications-Reaction Conditions Determine Surface Character". Platinum Met. Rev., 29, 175-179 (1985). [ Links ]
8. Figlarz, M., F. Fievet and J.P. Lagier, "Preparation of controlled size metal powders in micronicad sub-micronic range. A new process from polyol solutions", MRS Int'l Mtg on Adv. Mets. of Materials Research Society, 3, 125-129 (1989). [ Links ]
9. Garrels, R.M. and C.L. Christ, Solutions, Minerals and Equilibria, Ed. Freeman, Cooper & Company, San Francisco California (1965). [ Links ]
10. Habashi, F. "Principles of Extractive Metallurgy, Hydrometallurgy". Science Publishers, París. 2, 227. (1970). [ Links ]
11. Hedley, N. and H. Tabachnick, Chemistry of Cyanidation, Mineral Dressing Note American Cyanamid Company. New York, 23-29 (1958). [ Links ]
12. Lang, Lang's Handbook of Chemistry, 13th Spanish edition, II, 4-67. (1989). [ Links ]
13. Levenspiel, O. Ingeniería de las Reacciones Químicas. 2ª edición, editorial Reverte S.A., México, D.F. 8-15, 30-35. (1987). [ Links ]
14. Logan, S.R.. Fundamentos de Cinética Química. 1era Edición en Español Addison Wesley. 12-20. (2000). [ Links ]
15. Maron. S.H. and C.F. Prutton, Fundamentos de Fisicoquimica. Ed. Limusa, Mexico, D.F. (1974). [ Links ]
16. Matijevic, E. and W.P. Hsu, "Optical properties of monodispersed hematite hydrosols", Appl. Optics of Optical Society of America, 24, 1623-1630 (1985). [ Links ]
17. Metz, C.R.,.Fisicoquimica,Teoria y Problemas, Schaum Mcgraw-Hill, 96. (1976). [ Links ]
18. Pérez, L. "Curso de ciencia de los nuevos Materiales". Instituto de Materiales y Reactivos para la Industria Electrónica (IMRE), La Habana, Cuba (1995). [ Links ]
19. Perez-Robles, J.F., M.V. Méndez, L.M.R.A. Arellano, J.G. Hernandez and A.M. Ramírez, Mexican Patent No. 012067 (2001). [ Links ]
20. Sergueiev. Métodos Experimentales de la Cinética Química. Editorial Mir, Moscu. (1975). [ Links ]
Received: August 26, 2005.
Accepted for publication: June 22, 2006.
Recommended by Editor J. Pinto.














