Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Latin American applied research
versión impresa ISSN 0327-0793
Lat. Am. appl. res. v.37 n.2 Bahía Blanca abr. 2007
Phase transformations in clays and kaolins produced by thermal treatment in chlorine and air atmospheres
J. A. González, A. C. Carreras and M. del C. Ruiz*
Instituto de Investigaciones en Tecnología Química, Universidad Nacional de San Luis-CONICET
Chacabuco y Pedernera, c.c. 290, 5700 San Luis, Argentina
E-mail: mruiz@unsl.edu.ar
Abstract — A study of the phase transformations generated by chlorine during the calcination of clays and kaolins is presented. The original samples and residues of the thermal treatment carried out in air and chlorine atmospheres are analyzed at different calcination times and temperatures. Sample characterization was performed by X-ray diffraction (XRD), scanning electron microscopy (SEM) and X-ray microanalysis (EPMA). The results indicate that in samples of kaolin and ball clays the α -alumina phase (corundum) appears in the first chlorination stage. This phase disappears at longer chlorination times due to the volatilization of AlCl3. It is also observed that the kaolinite calcination in chlorine atmosphere favors the formation of the mullite phase. Other crystalline phases present in minerals, such as anatase and iron oxides, practically disappear after the samples chlorination.
Keywords — Kaolins. Clays. Chlorination. Calcination. Phase Transformations.
I. INTRODUCTION
The term kaolin is used to designate the white clays whose principal mineral is kaolinite (Al2Si2O5(OH)4). Its particles are usually hexagonal with diameters ranging from 0.05 to 10 m m (average 0.5 m m). Since this mineral is a product of the decomposition of feldspars and micas present in pegmatites and micaceous schists, it is frequently found together with other minerals such as quartz, sulfides, feldspars, micas and iron and titanium oxides, among others (Norton, 1983).
Kaolin is used for different industrial applications due to its physical and chemical properties. It is mainly used in the paper industry (45 %), refractories and ceramics (31 %), fiberglass (6 %), cement (6 %), rubber and plastic (5 %), paint (3 %) and others (4 %) (Murray, 2002).
Kaolinite is also the principal mineral of ball clays, but it exhibits lower granulometry and crystallinity than the kaolin. Kaolinite is usually accompanied by other clay minerals such as montmorillonite, illite, etc. Clays contain organic matter, generally lignite, and other colloidal minerals that often provide typical colorations. Since red clays possess a high content of iron oxide, they usually contain a higher concentration of titanium accompanying the iron that is generally found as ilmenite (Norton, 1983).
Iron is the principal contaminating agent in clays and kaolin. The presence of this element has a negative effect due to the color it gives to the product. Thus, the removal of iron from the kaolin used in industries, such as the paper industry, is of particular importance due to the requirements of high purity.
Numerous physical and chemical methods of iron extraction from these minerals have been investigated. The physical methods use separation techniques such as the flocculation and the high gradient magnetic separation (Chandrasekhar and Ramaswamy, 2002; Maury and Dixit, 1990). The chemical methods make use of leaching agents or leaching plus reductant agents (Ambikadevi and Lalithambika, 2000; Vèglio and Toro, 1994 and Atkinson and Fleming, 2001). Nowadays, leaching methods are being investigated using microorganisms (De Mesquita et al., 1996; Lee et al., 2002 and Cameselle et al., 2003).
The use of chlorination in the procedures of the extracting metallurgy has notably increased in the last decades, and a future increase in the use of chlorine in pyrometallurgic processes can be anticipated. This increase is due to numerous factors that include the high reactivity, the low cost, the variety and availability of chlorinating agents, the development of materials resistant to corrosion and the facility with which the effluents can be treated and recovered (Jena and Brocchi, 1997).
The advantages of chlorination in the removal of iron and titanium from clay and kaolin minerals used for the paper and ceramic industries in Argentina were studied in a previous work (González and Ruiz, 2006). It was observed that, at high temperatures, chlorine not only removes iron and titanium quantitatively by the formation of the gaseous FeCl3 and TiCl4 species, but also produces phase changes in the chlorinated minerals (González et al., 2003). The purpose of this work is to study the effect of chlorine on the phase transformations of clays and kaolins during calcination in chlorine atmosphere.
II. EXPERIMENTAL
A. Materials
The materials studied in this work were: high purity kaolin, provided by the Brazilian company CADAM and used in the argentine paper industry, and ball clay, provided by the argentine company Piedra Grande and used in the fine ceramic industry. 99.5 % pure chlorine (Indupa, Argentina) and 99.9 % pure nitrogen (AGA, Argentina) were used in the different assays.
B. Equipments
Figure 1 shows a diagram of the experimental equipment used in the chlorination assays. These assays were carried out in a quartz tubular reactor. The calcination in air was carried out in a conventional furnace.
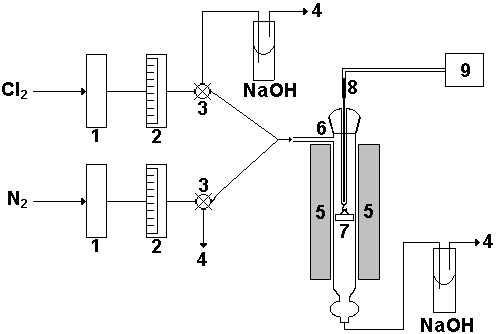
Fig. 1. Diagram of the experimental equipment. 1: H2SO4 desiccators, 2: flow-meters, 3: three-way valves, 4: venting, 5; electric furnace, 6: quartz reactor, 7: sample holder, 8: thermocouple, 9: thermometer.
Samples were analysed by X-ray diffraction (XRD), scanning electron microscopy (SEM) and X-ray microanalysis (EPMA). XRD studies were performed on a Rigaku D-Max IIIC diffractometer, using Cu-Kα radiation (l =0.15418 nm) with a Ni filter, and operated at 40 kV and 30 mA. SEM analysis was performed using a LEO 1450 VP microscope, equipped with an EDAX Genesis 2000 energy-dispersive x-ray analysis system (EDS), with a Si(Li) detector.
C. Procedure
The mineral sample subjected to chlorination was placed in a crucible and calcined in atmosphere of chlorine gas with a flow of 10 ml/min and at atmospheric pressure. The heating program was of 5oC /min from the room temperature to the working temperature. Once this temperature was reached, the sample was maintained in a chlorine gas flow as long as the experiment lasted. When the heating was finished, a purge was carried out with N2, and the sample was let to cool inside the reactor. The calcination in air was performed in a conventional furnace with the same heating program.
The characterization of the different samples was carried out by the X-ray diffraction in order to detect the crystalline phases present in the untreated samples and after undergoing different treatments. SEM techniques were used to study the changes in the particles morphology and in the crystals habits provoked by the thermal treatment in air and chlorine atmospheres. The EDS analysis was performed in order to study the composition of some particles and grains observed through SEM. For this, was suitable a standardless-analysis approach that permitted to estimate relative elemental concentrations and to establish their correspondence with the crystalline phases detected by XRD.
III. RESULTS AND DISCUSSION
A. Kaolin analysis
Sample without thermal treatment
The XRD analysis of the kaolin mineral without previous thermal treatment is shown in Fig. 2, in which it can be observed that the mineral is highly purified since only the peaks corresponding to the kaolinite phase are emphasized.

Fig. 2. X-ray diffractogram of untreated kaolin.
Figures 3a and 3b show SEM micrographs of particles of untreated kaolin, and Figure 3c shows an typical EDS spectrum of particles.

(a)

(b)

(c)
Fig. 3. (a) and (b) SEM micrographs and (c) EDS spectrum of pure, untreated kaolin particles.
The sample consists of approximately spherical particles with diameters ranging from 10 to 100 m m. The particle in Fig. 3a is formed by the agglomeration of small grains of sizes smaller to 1 m m, with the laminar structure characteristic of these materials. The EDS analysis showed signals corresponding to aluminium, silicon and oxygen as the constituting sample elements. The semi-quantitative analysis in atomic percentages of these three elements, normalized to 100 %, was the following: O: 59.97%; Si: 13.74% and Al: 12.84%.
This composition is closely correspondent to the typical kaolinite Al2O3*2SiO2*2H2O stoichiometry. In the spectrum shown in Fig. 3c, it can be observed that there are no peaks corresponding to elements different from those present in this phase, except for the gold peak, which corresponds to the metalizing in the sample, and the carbon peak, which is a normal contaminating agent of these materials.
Samples thermally treated
Figures 4a, 4b, 4c and 4d show the diffractograms of the kaolin samples subjected to calcination in air and chlorine at different temperatures and times.




Fig. 4. Diffractograms of the samples of kaolin calcined in Cl2 and air atmospheres. (a) at 900o C, 2 hs, (b) at 980oC, 2 hs, (c) at 980oC, 8 hs and (d) at 1050oC, 8 hs.
Some differences between the samples calcined in air and in chlorine flow can be observed in these figures: (a) the TiO2 anatase phase clearly appears in the samples calcined in air, but it does not appear in those calcined in chlorine due to the fact that titanium is removed from the sample by chlorination. Although species TiO2 was observed to be highly resistant to chlorine, with chlorination starting in an appreciable manner above 1400 oC, the presence of organic matter in clay minerals is an important factor in chlorination with chlorine gas, because carbon takes part in the chlorination reactions combining with the oxygen of the oxide and thus thermodynamically favoring the reaction. Also, carbon has kinetic effects on carbochlorination reactions. This phase is not detected in the sample of untreated kaolin probably due to its fine dispersion. (b) Chlorine induces the formation of the α -alumina phase of Al2O3 (corundum) at temperatures ranging from 900 to 980o C and at short calcination times. This oxide starts to be chlorinated at longer times and higher temperatures and it disappears at 1050oC, after 8 hours of treatment in chlorine atmosphere. The α -Al2O3 phase, characterized by its great hardness, probably forms from small quantities of the aluminum hydrated oxides, such as diaspore Al2O2(OH)2, bohemite Al2O2(OH)2, gibbsite Al2(OH)6 and amorphous hydroxide, which usually accompany kaolin minerals in very low proportions (Avgustinik, 1983). (c) Kaolinite turns to a metakaolinite phase due to the thermal treatment, and then, the mullite phase appears in more severe calcination conditions. The formation of the mullite phase, Al6Si2O13, necessary for the ceramic materials, is favored in chlorine atmosphere, especially at high temperatures. This fact is clearly evident in Fig. 4d, in which a significant increase of the crystallinity of the sample calcined in chlorine at 1050oC can be observed.
Figures 5a and 5b show micrographs of the residues of the kaolin samples calcined in chlorine and air, respectively, at 980oC for 2 hours. When comparing these micrographs with those shown in Figs. 3a and 3b, no significant changes in the samples morphology can be observed. There are no notable modifications in the particles topography, in the grains size or in the crystals habits. The anatase, α -alumina and mullite phases could not be identified by SEM-EDS, but their presence is clearly made evident by the corresponding XRD analysis.

(a)

(b)
Fig. 5. SEM micrograph of residues of kaolin calcined (a) in Cl2 and (b) in air, at 980oC for 2 hs.
B. Analysis of ball clay
Sample without thermal treatment
The XRD analysis of the untreated ball clay is shown in Fig. 6, in which the presence of the kaolinite, illite, quartz and albite feldspars phases can be observed.

Fig. 6. X-ray diffractogram of the untreated white clay.
Figures 7a and 7b show a SEM micrograph of a particle of untreated ball clay and its EDS spectrum, respectively. This particle is formed by the agglomeration of small grains of laminar shape ranging between 1 and 2 m m. The relations among the atomic concentrations of the elements detected (Al/O=0.19, Si/O=0.21 and Al/Si=0.90), obtained by EDS analysis, confirm that it is a kaolinite particle.

(a)

(b)
Figure 7. (a) SEM micrograph and (b) EDS spectrum of a particle of untreated ball clay.
Figures 8a and 8b show micrographs of particles, such as quartz and feldspar, respectively, identified by the EDS analysis.

(a)

(b)
Fig. 8. SEM micrographs of particles of (a) quartz and (b) feldspar, present in the untreated ball clay.
Samples thermally treated
Figures 9a, 9b, 9c and 9d show the diffractograms of the residues of the ball clay samples thermally treated in air and chlorine atmospheres, at different temperatures and calcination times. These diagrams are similar to those corresponding to kaolin. The anatase and rutile phases appear in samples calcined in air at 980oC. The α -Al2O3 phase appears in samples calcined in chlorine at 980oC after two hours of treatment. The formation of mullite is also favored by the thermal treatment in chlorine atmosphere, at higher temperatures and longer calcination times. Quartz is not affected by the thermal treatment in calcination conditions.




Fig. 9. Diffractograms of the ball clay samples calcined in Cl2 and air atmospheres, (a) at 900oC, 2 hs, (b) at 980oC, 2 hs, (c) at 980oC, 8 hs and (d) at 1050oC, 8 hs.
Figures 10a and 10b show a micrograph and the EDS spectrum of a particle belonging to a sample of ball clay calcined in air at 980oC. This particle presents a slight sintering of the grains without significant changes in the morphology or habit of the crystals. This phenomenon was observed in all the samples calcined in air, within the studied temperature range. The EDS spectrum shows the presence of O, Al and Si which are elements present in the crystalline phases observed by XRD or in the amorphous phases generated by the thermal treatment.

(a)

(b)
Fig. 10. (a) SEM micrograph and (b) EDS spectrum of a particle of ball clay calcined in air at 980oC.
Figures 11a and 11b show micrographs of the residue of the clay calcined in Cl2 atmosphere at 980oC, and Fig. 11c shows the EDS spectrum of one of the observed crystals. As shown by the results of the SEM analysis, a notable change in the particles morphology is observed. The grains evidenced a greater sintering, and needle-like crystals corresponding to the mullite phase appear. The relations between the atomic concentrations of the elements present in those needle-like crystals (Si/Al=0.30, Si/O=0.14 and Al/O=0.47) obtained by EDS reproduce the stoichiometry of the mullite phase. The presence of the mullite phase with these characteristics, which were not observed during the thermal treating of kaolin, might be due to the crystals formation originated in the gaseous phase through a process of recrystallization among the silicon chlorides, aluminum and oxygen, all of which result from the previous chlorination of the mineral. This phenomenon has been observed in the chlorination of refractory oxides such as Ta2O5 and Al2O3 in similar working conditions (González et al., 1998 and 2001; Lopazo and Pasquevich, 1997). However, this transformation of phases may occur through other ways that need to be investigated by carrying out new experiments.

(a)

(b)

(c)
Fig. 11. (a) and (b) SEM micrographs and (c) EDS spectrum of the residues of clay calcined in Cl2 atmosphere at 980oC.
V. CONCLUSIONS
The chlorination of ball clay and kaolin minerals, in dust as well as in ceramic pieces, has an advantage over the calcination in air: the formation at low temperatures of crystalline phases, such as the mullite, necessary for the ceramic materials.
Besides, the chlorination permits to remove iron and titanium impurities from those minerals. The appropriate control of the working conditions permits to achieve pure and notably white products.
The XRD, SEM and EPMA techniques used in this work to study the changes produced by the thermal treatment of white clays and kaolins appear to be adequate for this purpose.
ACKNOWLEDGEMENTS
The authors wish to thank the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) and the Universidad Nacional de San Luis for financial support.
REFERENCES
1. Ambikadevi, V.R. and M. Lalithambika, "Effect of organic acids on ferric iron removal from iron-stiend kaolinite" Applied Clay Science, 16, 133-145 (2000). [ Links ]
2. Atkinson, M.L. and M.E. Fleming, "Methods for bleaching kaolin clay and other minerals and bleached products obtained by the method", Patent EP1146089 (2001). [ Links ]
3. Avgustinik, A.V., Cerámica, Editorial Reverté, Barcelona (1983). [ Links ]
4. Cameselle, C.,M. T. Ricart, M.J. Núñez and J.M. Lema, "Iron removal from kaolin. Comparison between "in situ" and "two-stage" bioleaching processes", Hydrometallurgy, 68, 97-105 (2003). [ Links ]
5. Chandrasekhar, S. and S. Ramaswamy, "Influence of mineral impurities on the properties of kaolin and its thermally treated products", Applied Clay Science, 21, 133-142 (2002). [ Links ]
6. De Mesquita, L.M.S., T. Rodrigues and S.S. Gomes, "Bleaching of Brazilian kaolins using organic acids and fermented medium", Minerals Engineering, 9, 965-971 (1996). [ Links ]
7. González, J., M. Ruiz, J. Rivarola and D. Pasquevich, "Effects of heating in air and chlorine atmosphere on the crystalline structure of pure Ta2O5 or mixed with carbon", J. of Materials Science, 33, 4173-4180 (1998). [ Links ]
8. González; J., M. Ruiz, D. Pasquevich and A. Bohé, "Formation of niobium-tantalum pentoxide orthorhombic solid solutions under chlorine-bearing atmospheres", J. of Materials Science, 36, 3299-3311 (2001). [ Links ]
9. González, J.A., E. Perino and M. Ruiz, "Blanqueado de arcillas mediante la eliminación de hierro en la etapa de quemado", Proceedings Jornadas SAM-CONAMET, Simposio Materia, San Carlos de Bariloche, Argentina, T09-10 (2003). [ Links ]
10. González, J.A. and M. del C. Ruiz, "Bleaching of kaolins and clays by chlorination of iron and titanium", Applied Clay Science, 33, 219-229 (2006). [ Links ]
11. Jena, P.K. and E.A. Brocchi, "Metal extraction through chlorine metallurgy", Mineral Processing and Extractive Metallurgy Review, 16, 211-237 (1997). [ Links ]
12. Lee, E.Y., K-S. Cho and H.W. Ryu, "Microbial refainement of kaolin by iron-reducing bacteria", Applied Clay Science, 22, 47-53 (2002). [ Links ]
13. Lopasso, E.M., J.J. Andrade Gamboa, J.M. Astigueta and D.M. Pasquevich, "Enhancing effect of Cl2 atmosphere on transition aluminas transformacion", J. of Materials Science, 32, 3299-3304 (1997). [ Links ]
14. Maurya, C.B. and S.G. Dixit, "Effect of pH on the high-gradient magnetic separation of kaolin clays", International Journal of Mineral Processing, 28, 199-207 (1990). [ Links ]
15. Murray, H., "Industrial clays case study", Mining, Minerals and Sustainable Development, 64, 1-9 (2002). [ Links ]
16. Norton, F.H., Cerámica Fina, Editorial Omega, Barcelona (1983). [ Links ]
17. Vegliò, F. and L. Toro, "Process development of kaolin pressure bleaching using carbohydrates in acid media", International Journal of Mineral Processing, 41, 239-255 (1994). [ Links ]
Received: March 21, 2006.
Accepted for publication: August 2, 2006.
Recommended by Editor J. Pinto.














