Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Latin American applied research
versão impressa ISSN 0327-0793
Lat. Am. appl. res. v.37 n.4 Bahía Blanca out. 2007
Photopolymerisation of acrylic acid and chitosan gels (I). Influence of preparation method on the formation and kinetic behaviour of interpenetrating complexes
N. Davidenko1, C. Peniche1, J. M.Díaz2 J. San Roman2 and R. Sastre2
1 Centro de Biomateriales, Universidad de la Habana, P.O.Box 10400, Havana, Cuba
natalia@biomat.uh.cu; peniche@reduniv.edu.cu
2 Instituto de Ciencia y Tecnología de Polímeros, CSIC, Juan de la Cierva 3, 28006- Madrid, Spain
jsroman@ictp.csic.es; rsastre@ictp.csic.es
Abstract — The kinetic study of the photopolymerisation of gels of acrylic acid (AA) and chitosan (CHI) prepared by three different methods was accomplished. The kinetic parameters such as conversion and polymerisation rate were determined. The influence of the composition and the preparation method on these parameters was elucidated. The effect of the mixing order on the formation of chitosan-acrylic acid complexes (CHI+AA-) was determined by measuring the viscosity of non-polymerised gels. The presence of polyacrylic acid (PAA) grafted on to a CHI matrix after photopolymerisation and the influence of the method of preparation and feed composition on the proportion of CHI-PAA interpolymer network were demonstrated by FTIR spectroscopy. The possible mechanism of the graft copolymerisation is discussed in detail.
Keywords — Acrylic Acid. Chitosan. Interpolymer Complexes. Gels. Photopolymerisation.
I. INTRODUCTION
The preparation of hydrogels based on the intimate mixture of natural or synthetic macromolecules and polymer chains formed by polymerisation reaction in the presence of the macromolecular template has received increasingly growing attention in the past few decades. The macromolecular components of these hydrogels generally possess the capacity for forming between themselves the interpolymer complexes through electrostatic interactions, hydrogen bonds and Van der Waals forces, etc. Among the synthetic vinyl-like monomers the acrylates, acrylamides and acrylonitriles are the most widely used in these systems. These structures are capable of interacting with amine and hydroxyl groups of some polysaccharides to form corresponding complexes which can be polymerised by a free radical route .
In the family of natural polymers, chitosan, CHI, is one of the most extensively employed for this purpose. It is worth mentioning that CHI itself has attracted great interest in medicine and pharmacy because of its biodegradability, biocompatibility and the wide range of positive biological responses and activities reported. It has been used in or proposed for the production of drug delivery devices, cell encapsulation , as a wound healing agent, in adhesive formulation for surgical applications, in ophthalmology and dentistry, etc.
It is also fungi- and bacteriostatic. In addition, this highly basic polysaccharide displays a number of ionisable primary amino groups which are readily available for chemical reaction and salt formation with acids including synthetic anionic monomers and macromolecules [interpenetrating networks (IPN), polyelectrolyte complexes, graft copolymers, blends and others]. The complex CHI-polyacrylic acid (PAA) is one of the most studied. This type of hybrid system can be obtained by mixing polymeric aqueous solutions but also by the so-called template polymerisation, which involves the polymerisation of the anionic monomer (acrylic acid) in the presence of chitosan. Cross-linking of these complexes with glutaraldehyde or by thermal treatment gives rise to a complex/semi-IPN structure by amide formation.
There are some reports of the use of chitosan/PAA-based matrices obtained by template polymerization for mucosal delivery systems and as drug carriers. In our laboratory we have prepared chitosan/ PAA matrices by template polymerization at low temperature by taking advantage of activation of the decomposition of the initiator by chitosan . It was also found that during polymerization under these mild conditions some grafting of polyacrylic acid (PAA) chains on to chitosan molecules was produced, giving rise to weakly crosslinked hydrogels after neutralization. These hydrogels exhibited water uptakes as high as 560 g of water per g of dry sample.
It is important to stress, that the majority of CHI/ PAA interpenetrating systems were produced by initiation of template polymerisation through thermal or red-ox routes, there being few reports about the possibility of photochemical initiation for these systems. However, it is well known that photochemical polymerisation techniques possess an important advantage, such as the unlimited and controlled manipulation time which makes the photo-cured system very attractive for several medical applications.
For this reason the objective of the current work has been to study the kinetics of this novel procedure of preparation of macromolecular hydrogels based on the intimate mixture of natural polysaccharide (CHI) and PAA chains formed by radical photopolymerisation of AA in the presence of the mentioned macromolecular template. This study involves the determination of some kinetic parameters such as double bond conversion and polymerisation rates at different reaction times. In addition, in order to determine the suitability of these systems as potential biomaterials the influence of the feed mixture composition and the preparation procedure for these gels on the formation of polyelectrolyte complexes and IPN were clarified.
II. EXPERIMENTAL
A. Materials
Chitosan [Deacetylation Degree (D.D.)=79.9% determined by 1H-NMR, Mv=2x105] was obtained from shells of lobsters (Panulirus argus) as described elsewhere . For their purification the Chitosan flakes were dissolved in aqueous acetic acid (1 wt.-%), filtered and precipitated with aqueous NaOH (40 wt.-%). The precipitated gel was washed several times with water and vacuum dried in a desiccator. The white chitosan foam was ground to obtain a powdered material with grain size in the range of 50-200 μm. The material thus obtained was stored in a closed flask until required.
Acrylic acid (MERCK) and Camphorquinone (CQ) (Aldrich) were used without further purification. 4-Dimethylaminobenzyl alcohol was synthesized as described elsewhere and used as co-initiator.
B. Preparation of gels
The gels of AA and CHI were prepared by the methods described schematically in Fig. 1. In method I a fixed volume of distilled water was added to a measured quantity of CHI and this mixture was allowed to rest for about 10 minutes, so that the swelling of CHI was completed. The quantity of water added was equal to the volume of AA to be added later. 1% by weights of both CQ and DMOH as initiation system were firstly added to the monomer AA.
Fig. 1. Gel preparation methods.
In method II the order of addition of AA and water was varied, that is to say, firstly the CHI and the AA (containing initiation system) were mixed and then the water was added to this mixture.
In method III firstly the AA (containing the initiation system) was diluted in water and later a predetermined quantity of CHI was added to this solution.
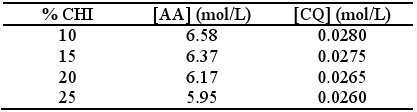
Independent of the method of preparation, these gels were left in the dark to rest for 24 hours before use to ensure that equilibrium between AA and CHI interaction was achieved. By measurement of pH it was previously shown that equilibrium was reached three hours after preparation. The compositions of the prepared gels are shown in Table 1. The ratio AA:H2O was constant and equal to 50:50.
Table 1. Composition of gels.
C. Photopolymerisation kinetics
The photopolymerisation kinetics were monitored by differential scanning photocalorimetry. The experimental system used in this work consisted of a conventional Perkin-Elmer DSC-7 differential calorimeter suitably modified to permit irradiation of the samples within the calorimeter chamber. A detailed description of this system is found in previous publications . In our current work we used Schott KL-1500 as a light source which allows the direct regulation of the light intensity in graduated stages by means of a brightness control. Installation of neutral filters in the filter holder, or on the focusing attachment of the instrument, extends the range of intensity control. In order to avoid the heating effect of the photocuring source and to isolate the 470 nm wavelength, a solid heat filter (Schott KG-1) and a 470 violet filter (Schott VG-9) were placed into the filter holder.
The DSC calibration, data processing, determination of incident and absorbed light intensity, as well as complementary details, have been described elsewhere . All polymerisations were carried out at a temperature of 37°C, being similar to that of the human body. Sample quantities between 5 and 7 mg were placed on the base of the 6.5mm diameter aluminium DSC pan fitted with a transparent PMMA cover so as to avoid the vaporization of the reaction mixture. The incident light intensity (Io) was maintained constant and equal to 0.26 mcal/sec.
The rate of polymerisation (mol/L.s) was calculated from the heat flow and the sample mass of AA, while the enthalpy of polymerization was assumed to be 13.6 kcal/mol for each acrylate group .
All values reported for the kinetic parameters correspond to the average of at least three measurements, with a maximum relative error of 3 %.
D. Viscometry
The measurements of non-polymerised gels were carried out in a Brookfield viscometer at a temperature of 25°C using an appropriate spindle. Previously the calibration of this equipment was made using glycerine of known viscosity. All the values of viscosity were taken 2 minutes after commencement of the measurement and the reported value corresponded to the average of at least three measurements with the error of about 3%.
E. FTIR spectra
In order to determine the FTIR spectra of completely polymerised gel films, a more powerful commercial source of irradiation was used. The films of 1mm thickness were irradiated for 3 min. with a UV-Visible Visilux 2, 3M lamp. The incident light intensity was 3mcal/s approximately. The polymerised films obtained were swollen in distilled water at 37°C for 24 hours to remove any soluble material. During this time the water was changed periodically. Afterwards the films were dried at 25°C under vacuum to constant weight.
In order to extract the non-grafted and soluble PAA the films were washed in an alkaline medium (NaOH, 5 wt.-% in water) for 3 days at room temperature and afterwards thoroughly washed with distilled water and dried under vacuum to constant weight.
Samples of 1 mg were finely ground with thoroughly dried KBr and discs were prepared by compression under vacuum. FTIR spectra were recorded on a Nicolet 520 spectrometer. Spectra were taken with a resolution of 2 cm-1 and were averaged over 32 scans.
III. RESULTS AND DISCUSSION
A. Kinetics of photopolymerisation
A comparative kinetic study of the photopolymerisation of the gels prepared by the three methods (gels I, II and III), was carried out with the objective of determining the influence of the CHI content and of the preparation method on the selected kinetic parameters.
In order to achieve a correct rate comparison, account was taken of the fact that while varying the content of CHI, the concentrations of both the monomer and the initiation system (CQ-amine) would also vary slightly.
The rates of polymerisation were therefore normalized for the concentrations of the standard monomer (6.58 mol/L) and for the initiation system (0.028 mol/L).
As an example, the kinetic curves obtained for gels II with different CHI content are shown in Fig. 2, these curves being similar to those obtained for the gels prepared by the other two methods. The profiles displayed suggest that in these systems the classical effect of auto-acceleration is not present, possibly due to dilution of the monomer.
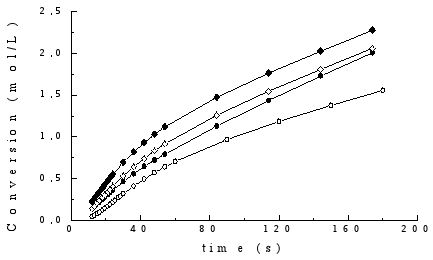
Fig. 2. Kinetic curves of polymerisation of gels prepared by method II. The corresponding chitosan contents (in wt.-%) are: ( ), 10; (
), 10; ( ), 15; (
), 15; ( ), 20; (
), 20; ( ), 25.
), 25.
The corresponding rates of polymerisation were calculated by a linear least-squares fit of the steady state regions of the curves of conversion vs time, where good correlation coefficients (between 0.9995 and 0.9999) were observed. The values of initial polymerisation rates (Rp´) are shown in Table 2. Results were normalized to [AA] = 6.58 mol/L and [CQ] = 0.028 mol/L.
Table 2. Comparison of the initial rate of polymerisation Rp´ of gels prepared by different methods.

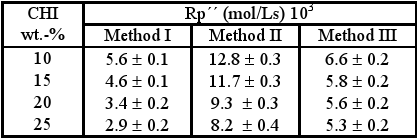
It can be observed that increasing the CHI content leads to increase in Rp´ for all preparation methods, these values being greater for gels obtained by method II.
Table 3 shows the results obtained for the rate of polymerization in the time interval ranging from 2 to 5 minutes irradiation time (Rp´´). It is noticeable that in all the cases studied the increase in CHI content leads to the diminution of rate (Rp´´), possibly due to the vitrification of the reaction media at conversions above 20% which can cause a decrease of the propagation constant of the process of polymerization, kp. By comparing the rates Rp´´ for these different preparation methods, it can be observed that the greatest values are reached for the gels obtained by the method II.
Table 3. Results obtained in determination of Rp´´.
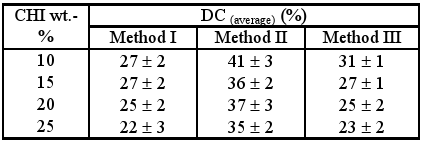
On the other hand, the degree of conversion calculated after 4 minutes irradiation (Table 4) also shows higher values for gels II while for all methods a decreasing trend is observed with the increase of CHI content.
Table 4. Degree of conversion (DC, %) after 4 minutes irradiation.
To explain these results it is necessary to consider the influence of the method of preparation and the CHI content on the characteristics of these systems such as viscosity and strength of interactions between the main components, CHI and AA, which lead to the formation of the interpolymeric complex.
B. Viscosity of non-polymerised gels
It is evident that the viscosity of a system should strongly influence the kinetic parameters of the process of polymerisation, since it is clear that with the increase of viscosity the mobility of the macro-radicals diminish, which, in turn, affects the constant of termination, kt. This leads to an increase of kp/kt1/2 ratio and, consequently, to an increase in the rate of polymerisation.
On the other hand, at high conversions the opposite effect can take place due to the vitrification of the reaction media. This would affect the propagation constant, kp, and, as a consequence, diminish the rate of the polymerisation process.
The results obtained in the measurement of viscosity of non-polymerised gels are presented in Table 5.
Table 5. Viscosity of gels with different CHI contents. 
(-) Values of viscosity exceed the upper limits of the technique used.
As may be observed, the increase of only 5 wt.-% in CHI content results in an abrupt increase in the viscosity of the system, leading to differences of more than an order of magnitude, gels II being much more viscous in comparison with those prepared by the other methods. To explain these differences we presumed that in method II, where the main components of this system (AA and CHI) come into direct contact, the interactions between the NH3+ groups of CHI and the carboxyl groups of AA are favoured, leading to a increased formation of a polyelectrolyte complex .
C. FTIR study of the structure of the polymerised gels
As mentioned previously, the differences in the CHI content and in the methods of gel preparation can influence both the interactions between AA and CHI and the ultimate formation of grafting PAA on to the CHI polymeric matrix. To clarify this question an FTIR study of photopolymerised gels obtained by different methods was carried out.
Figure 3 shows the IR spectra of CHI (a), PAA (c) and polymerised gel (b) prepared by method II with 25 wt.-% CHI after having been washed thoroughly to extract non-reacted AA and free (non-grafted and soluble) PAA. The spectra of the other polymerised gels are qualitatively similar to that of (b) but are not shown for the sake of simplicity. In all of these spectra the bands at 1553 cm-1 (asymmetrical vibrations of carboxyl anion -COO-) and 1405 cm-1 (symmetrical -COO- stretching) are observed, revealing the presence of this anion in the structure of the copolymer obtained.

Fig. 3. Infrared spectra in the region 1000-2000 cm-1·for chitosan (a), poliacrylic acid (c) and (b) the gel obtained by method II.
On the other hand, the bands at 1720 cm-1, corresponding to the carbonyl group (-COOH) of the free polyacrylic acid (PAA), and at 1082 and 1030 cm-1, characteristic of the CHI saccharide structure , are present, which indicate that grafting copolymerization of PAA on to the CHI matrix has taken place.
As only free PAA and that grafted to the CHI matrix can contribute to the intensity of the band at 1720 cm-1 this band may be used to indicate the proportion of PAA in the gel sample. The presence of -COO- anion provides evidence of the existence of a polyelectrolyte complex (CHI+-PAA) formed by interaction of amino groups of CHI with the carboxylic group of PAA .
The band at 1082 cm-1 is characteristic of skeletal vibration of CHI and should increase in intensity with the increase of CHI content in the gel. Therefore, the ratio A1720/A1082 allows the relative quantity of chitosan in the polymerised gels to be evaluated . In the results shown in Fig. 4, it can be seen that, with the increase in CHI content of the feed, the value of the ratio A1720/A1082 is diminished for the polymerised gels prepared by the three methods. This tendency is linear in the composition interval studied.
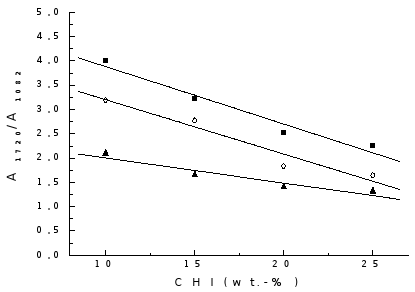
Fig. 4. Variation of A1720/A1082 with CHI content: ( ) Method I; (
) Method I; ( ) Method II; (
) Method II; ( ) Method III.
) Method III.
When comparing preparation methods it can be seen that for the gels obtained by method II this ratio is lower than for other methods, indicating that the quantity of grafted PAA is smaller.
It has already been established that during the polymerization reaction of the complex monomer-chitosan system, variable quantities of graft copolymers can be formed by two different mechanisms .
-
The amidation between the free carboxylic group of the acrylic acid (COO-) and the amine functions of the chitosan, which provide the grafting of the polyacrylic chains on to the chitosan macromolecules:
|-COO-++H3N-| →|-CONH-|+H2O - Transfer reaction of the growing polymeric radicals with both the -NH2 and -OH functional groups of the pyran chitosan rings
These two mechanisms can explain the three-dimensional network structure of these complex systems after the polymerisation reaction.
There is also, however, the possibility of grafting the PAA chain into the CHI skeleton by means of the initiation of polymerisation of the AA with the amino-radicals obtained from CHI. The formation of this type of radical during the decomposition of charge transfer complexes, formed between the amine groups of CHI and certain electron acceptors, has been reported in another work .
In the case of photoinitiation it has been demonstrated that CHI can photo-reduce the CQ and form the corresponding exiplex between excited CQ and CHI whose decomposition gives rise to the amino radicals which, in turn, can initiate the process of polymerisation of the AA. In this way the PAA chains can be linked to the CHI matrix forming semi-interpenetrating copolymer system:
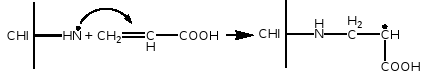
If the formation of grafting occurs for mechanism 1, that is to say, through interaction of the ionic groups COO - and NH3+ the copolymers obtained from more viscous gels (method 2) should have a greater quantity of grafted PAA since in this system (with more electrostatic interaction) this type of ionic bonding between AA and CHI should be favoured in comparison to the others methods of preparation. However, the results obtained from the IR study of polymerised gels contradict this supposition by showing a greater quantity of grafted PAA in the less viscous gels obtained by the method 3. So, it is logical to suppose that grafted copolymer is formed fundamentally either by the mechanism 2 (through the transfer of the growing radical with the amino or hydroxyl groups of CHI ring) or through the initiation of the polymerisation of AA by CHI-amino radicals. The fact that the grafting content follows an inverse tendency to the viscosity of the system before polymerising supports this supposition. For the more viscous gels (method II) the transfer reactions should be disfavoured due to the decrease in mobility of the growing macro-radicals (PAA) as well as CHI-type polymeric amino-radicals. In addition, the greater the quantity of amine groups involved in electrostatic reactions with AA forming the polyelectrolyte complex CHI+AA-, fewer remain available for the photo reduction of CQ giving rise to the formation of CHI amino-radical. In this way, by accepting the supposition that the formation of a grafted interpenetrated copolymer between CHI and PAA occurs through mechanism 2 or through reaction of AA with CHI amino radicals, the results of the viscosity study are in complete agreement with the results of IR spectroscopy.
On the other hand, it also seems logical to suppose that the AA included in the polyelectrolyte complex could possess different reactivity to the free acrylic acid, which would change the potential barrier of the monomer-polymer step. This, in turn, would be reflected in the activation energy of the polymerisation process. With the purpose of clarifying this question the determination of this parameter for the photopolymerisation of AA in presence and absence of CHI was carried out.
D. Determination of the Activation energy
The determination of the activation energy (Eact) was carried out by the Arrhenius method. In the specific case of photopolymerisation, where the generation of radicals takes place through a photochemical route without the need for a thermal contribution, the rate of polymerization, Rp´, depends on the propagation, kp, and termination, kt, constants according to the following equation:

|
(1) |
where φi is the initiation quantum yield, Ia is the absorbed light intensity and [M] is the monomer concentration.
Applying the Arrhenius equation to the ratio of the constants we obtain the following equation:
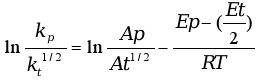
|
(2) |
where Ap and At are pre-exponential factors of propagation and termination, respectively, Ep - (Et/2) is an apparent activation energy of polymerization, Eact, assuming a mechanism with a bimolecular termination step and zero activation energy for photoinitiation.
Substituting the constant ratio kp/kt1/2 by its expression obtained from the previous equation (2) and introducing it in the equation (1) we will have:
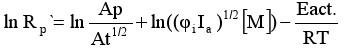
|
(3) |
Therefore, it is possible to calculate Eact as a slope of the ln Rp´ plot against the inverse of temperature.
For this study, the gels with 20% of chitosan content prepared by different methods were chosen in order to compare their activation energies with those obtained by the photopolymerisation of diluted acrylic acid (AA: H2O = 50:50).
For this purpose the temperature dependence of the polymerisation rates of these systems was established taking 5 experimental points in the interval between 25 and 40°C. This interval was selected since below 25°C the AA, with its relatively high crystallisation point (∼13.5°C), separates out. The Arrhenius plot of the data obtained (ln Rp´ vs 1/T) showed a linear dependence, and the slopes permitted the corresponding apparent activation energy of studied formulations to be determined. The results obtained are shown in Table 6.
Table 6. Activation Energy and Correlation coefficients (r) for the photopolymerisation of gels and diluted AA
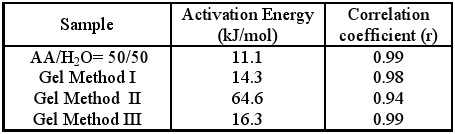
As may be observed, the activation energies obtained in the presence of CHI are greate in comparison with AA:H2O solution. This indicates that the existence of the polyelectrolyte complex CHI+AA- increases the potential barrier of the monomer-polymer conversion step. Reinforcing this supposition is the fact that in the case of gels II, where ionic interactions between the carboxylic and amine groups are favoured, an Eact value almost six times higher than in other methods is found. Therefore, the observed increase in the rates of polymerisation of the gels with a higher content of the polyelectrolyte complex (CHI+AA-), could not be explained on the basis of greater reactivity of this complex, but rather as a consequence of the increase in the viscosity of the reaction media. This results in a decrease in the corresponding termination constant (kt) and the consequent increase in the value of Rp´.
IV. CONCLUSIONS
The results of this work indicate that it is possible to prepare gels of AA/CHI that can polymerise through the photochemical route. The increase of CHI content leads to increase in viscosity of the reaction media due, fundamentally, to the formation of a polyelectrolyte complex. This complex results from the interaction between both speciesand is favoured in the case of the gels prepared by method II. It has been demonstrated that the formation of this polyelectrolyte complex influences the photopolymerisation kinetics of the gels: an increase of its content induces an increment in the initial rate of polymerisation, Rp´, due to increase in viscosity of the reaction media and consequent decrease of the termination constant (kt) of the reaction. At longer irradiation times the rate of polymerization, Rp´´ diminishes as a consequence of vitrification of the medium, which is also shown by the reduction in the degree of conversion (DC).
Furthermore, the results obtained from IR spectra of polymerised and washed gels demonstrate that the quantity of grafting PAA on to chitosan is smaller in the case of the gels II where, according to the method of preparation and to the higher viscosity, a greater number of ionic type interactions between AA and CHI should exist. This leads to the supposition that the formation of graft copolymer between PAA and CHI occurs through the mechanism that involves transfer reaction of growing polymeric radicals to functional groups of the pyran cycles of chitosan and not through the formation of interchain amide bonds between COO- groups of AA and NH3+ of CHI.
The results obtained for viscosity measurements of non-polymerised gels are in agreement with this supposition, since, according to the proposed mechanism, the graft formation should be disfavoured in method II which produces the most viscous gels and where the mobility of growing macro-radicals should be disfavoured. Also contributing to this conclusion is the fact that in the less viscous systems (gels I and III), where the mentioned transfer reactions of the growing PAA radicals should be favoured (because of their higher mobility), the greatest quantity of graft copolymers is observed.
REFERENCES
1. Ahn, J.S., H.K. Choi and C.S. Cho, "A novel mucoadhesive polymer prepared by template polymerization of acrylic acid in the presence of chitosan", Biomaterials, 22, 923-928 (2001). [ Links ]
2. Argüelles, W., and C. Peniche, "Study of the Interpolyelectrolyte Reaction Between Chitosan and Carboxymethyl Cellulose", Makrom. Chem., Rapid Commun., 9, 693-697 (1988). [ Links ]
3. Argüelles, W., and C. Peniche, "Preparation of a novel polyampholyte from chitosan and citric acid", Makrom. Chem., Rapid Commun., 14, 735-740 (1993). [ Links ]
4. Borzacchielo, A., L.A.A. Netti, L. Nicolais, C. Peniche, A. Gallardo and J.S. Román, "Chitosan-based hydrogels: synthesis and characterization", J. Mater. Sci: Mater. in Med., 12, 861-864 (2001). [ Links ]
5. Cook, W.D., "Photopolymerization kinetics of dimethacrylates using the camphorquinone/amine initiator system", Polymer, 33, 600-609 (1992). [ Links ]
6. Davidenko, N., D. Zaldívar, C. Peniche, R. Sastre and J.S. Román, "Activity of the furan ring in the free radical polymerization of acrylic monomers", J. Polym. Sci. Polym. Chem. Ed. Part A, 34, 2759-2766 (1996). [ Links ]
7. Davidenko, N., O. García and R. Sastre, "The efficiency of titanocene as photoinitiator in the polymerization of dental formulations", J. Biomat. Sci. Polymer Edn, 14, 733-746 (2003). [ Links ]
8. Davidenko, N., O. García and R. .Sastre, "Photopolymerization kinetics of dimethacrylate-based light-cured dental resins", J. Appl. Polym. Sci., 97, 1016-1023 (2005). [ Links ]
9. Elvira, C., B. Levenfeld, B. Vázquez and J.S. Román, "Amine activators for the cool peroxide initiated polymerization of acrylic monomers", J. Polym. Sci. Polym. Chem. Ed., 34, 2783-2789 (1996). [ Links ]
10. Gallardo, A., M.R. Aguilar, C. Elvira, C. Peniche and J.S. Román, "Chitosan Based Microcomposites - From Biodegradable Microparticles to Self-Curing Hydrogels", In R. Reis and J. S. Román, eds. Biodegradable Systems in Tissue Engineering. CRC Press, Boca Ratón, 145-162 (2005). [ Links ]
11. Hirano, S., H. Seino, Y. Akiyama and I. Nonaka, "Chitosan: a Biocompatible Material for Oral and Intravenous Administration", In C. G. Gebelein and R. L. Dunn, eds. Progress in Biomedical Polymers, 283. Plenum Press, New York, (1990). [ Links ]
12. Hoffman, A.S., "Hydrogels for biomedical applications", Advanced Drug Delivery Reviews, 43, 3-12 (2002). [ Links ]
13. Hu, Y., X. Jiang, Y. Ding, H. Ge, Y. Yuan and C. Yang, "Synthesis and characterization of chitosan-poly(acrylic acid) nanoparticles", Biomaterials, 23, 3193-3201 (2002). [ Links ]
14. Kurita, K., M. Kawata, Y. Koyama and S. Nishimura, "Graft copolymerization of vinyl monomers onto chitin with cerium (IV) ion", J. Appl. Polym. Sci., 42, 2885-2891 (1991). [ Links ]
15. Kurita, K., K.Tomita, T.Tada, S.Ishi, S.Nishimura and K. Shimada, "Squid chitin as a potential alternative chitin source: Deacetylation behavior and characteristic properties", J. Polym. Sci. Polym. Chem. Ed., 31, 485-491 (1993). [ Links ]
16. Lindén, L.A., In J. P. Fouassier and F. Rabek, eds. Radiation Curing in Polymer Science and Technology. Elsevier, London, 387-395 (1993). [ Links ]
17. Mateo, J.L., P. Bosch, F. Catalina and R. Sastre, "Novel dialkylaminoalkyl- and dialkylaminoalcoxy- benzophenones as polymerization photoinitiators. II. Photocalorimetric study on photoinitiated polymerization of butyl and lauryl acrylates", J. Polym. Sci. Polym. Chem. Ed.Part A: Polym. Chem., 30, 829-834 (1992). [ Links ]
18. Peniche, C., C. Elvira and J.S. Román, "Interpolymer complexes of chitosan and polyacrylic derivatives of salicylic acid: preparation characterization and modification by thermal treatment", Polymer, 25, 6549-6554 (1998). [ Links ]
19. Peniche, C., W. Argüelles, N. Davidenko, R. Sastre, A. Gallardo and J.S. Román, "Self-curing membranes of chitosan/PAA IPNs obtained by radical polymerization: preparation, characterization and interpolymer complexation", Biomaterials, 20, 1869-1878 (1999). [ Links ]
20. Peniche, C., W. Argüelles-Monal, H. Peniche and N. Acosta, "Chitosan: an attractive biocompatible polymer for microencapsulation", Macromol. Biosci., 3, 511-520 (2003). [ Links ]
21. Torre, P.M., G. Torrado and S. Torrado, "Interpolymer complexes of poly(acrylic acid) and chitosan: influence of the ionic hydrogel-forming medium", Biomaterials, 28, 1459-1468 (2003a). [ Links ]
22. Torre, P.M., Y. Enobakhare, G. Torrado and S. Torrado, "Release of amoxicillin from polyionic complexes of chitosan and poly(acrylic acid). study of polymer/polymer and polymer/drug interactions within the network structure", Biomaterials, 24, 1499-1506 (2003b). [ Links ]
23. Wang, H., W. Li, Y. Lu and Z. Wang, "Chitosan-based hydrogels. Synthesis and characterization", J. Appl. Polym. Sci., 65, 1445-1450 (1997). [ Links ]
24. Xu, H.H., J.B. Quinn, S. Takagi and L.C. Chow, "Processing and Properties of Strong and Non-rigid Calcium Phosphate Cement", J. Dent. Res., 81, 219-224 (2002). [ Links ]
25. Yazdani-Pedram, M., J. Retuert and R. Quijada, "Hydrogels based on modified chitosan, 1. Synthesis and swelling behavior of poly(acrylic acid) grafted chitosan", Macromol. Chem. Phys., 201, 923-930 (2000).
[ Links ]
Received: November 9, 2006
Accepted: March 1, 2007
Recommended by Subject Editor: Ricardo Gómez














