Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Latin American applied research
versión impresa ISSN 0327-0793
Lat. Am. appl. res. v.37 n.4 Bahía Blanca oct. 2007
Effect of operating conditions on Fischer-Tropsch liquid products
F. E. M. Farias, F. R. C. Silva, S. J. M. Cartaxo, F. A. N. Fernandes and F. G. Sales
Universidade Federal do Ceará, Departamento de Engenharia Química,
Campus do Pici, Bloco 709, 60455-760 Fortaleza - CE, Brazil
fabiano@deq.ufc.br
Abstract — Fischer-Tropsch synthesis is an important chemical process for the production of liquid fuels. In recent years, the abundant availability of natural gas and the increasing demand of gasoline, diesel and waxes have led to a high interest in further developing this process. The dependencies of liquid hydrocarbon product distribution of iron catalyzed Fischer-Tropsch synthesis on operating pressure and temperature have been studied. The study followed an experimental planning and the results were analyzed based on surface response methodology. The effects of different operating conditions on product distribution were compared based on number average carbon number and distribution dispersion. Results showed that high temperature (270°C) and pressure (27 atm) favor the production of heavy waxes that can be converted to liquid fuels through hydrocracking, while greater direct selectivity towards liquid fuels are favored by low temperature (240°C) and high pressure (30 atm).
Keywords — Fischer-Tropsch Synthesis. Liquid Fuels. Iron-based Catalyst.
I. INTRODUCTION
In recent years, the Fischer-Tropsch synthesis (FTS) has become a subject of renewed interest particularly in converting natural gas (NG) into liquid transportation fuels. This can became a major advantage when dealing with natural gas because its storage is a big logistic problem, especially regarding exploration in off-shore exploitation platforms. A palliative transportation solution, which has been in relatively widespread use for years involves the conversion of NG into liquefied natural gas (LNG). However the LNG approach has a significant drawback since it is a relatively expensive process and the storage vessels requires rigorous thermal design and construction specs due to the required low temperatures. For that reason, if the FTS process is further developed and performed efficiently, it may become an economically viable alternative for delivering natural gas across large distances.
Natural gas can be converted to carbon monoxide and hydrogen (synthesis gas) via the existing processes, such as steam reforming, carbon dioxide reforming, partial oxidation and catalytic partial oxidation, followed by the FT synthesis reaction:
CO + 2H2 → (-CH2 -)+ H2O (1)
The study of the Fischer-Tropsch synthesis (FTS) with iron-based catalysts has been done by many investigators (Raje and Davis, 1997; van Steen and Schulz, 1999; Donnelly and Satterfield, 1989; Eliason and Bartholomew, 1999; Li et al., 2002), and it has been shown that iron-based catalysts has satisfactory performance in the production of liquid fuels in the range of gasoline and diesel. Most researches have focused on the conversion of the FTS and on the overall rate of reaction (Raje and Davis, 1997; Eliason and Bartholomew, 1999) and many overall reaction rate equations of the FTS reaction have been proposed.
In recent years the focus on FTS researches have shifted towards a better understanding of the FT kinetic mechanism (Huff and Satterfield, 1984; Mandon and Taylor, 1981; Maitlis et al., 1999) and product distribution (Patzlaff et al., 1999; Donnelly and Satterfield, 1989; van der Laan and Beenackers, 1999; Fernandes, 2005, 2006; Fernandes and Sousa, 2006). Studies have shown that iron-based catalyst produces paraffins, especially low molecular weight paraffins (light gases, gasoline and diesel cuts), and a large quantity of olefins depending on the operating conditions that are employed.
van Berge (1997) has shown that in comparison to cobalt-based catalysts, iron-based catalysts present better performance and higher productivities than the Co-based catalyst at high pressures (over 10 atm, preferably above 20 atm) and space velocities, thus this evidence has to be further studied. In this study we have examined the number of carbon distribution of the liquid product of a FT reaction carried out under pressures between 20 and 30 atm, range of pressure which few papers are available in the literature.
II. EXPERIMENTAL
A. Catalyst Preparation
An iron catalyst with a 100Fe/5Cu/6K/139SiO2 composition (molar basis) was prepared by successive impregnation with aqueous solution of Fe(NO3)3.9H2O, Cu(NO3)2.3H2O and K2CO3 to incipient wetness of SiO2 (Grace-Davison Syloid 74) in a rotary evaporator (Tecnal model TE-211, Brazil) operated at 60°C under 500 mmHg (vacuum maintained with a vacuum pump Tecnal model TE-058).
After impregnation of each salt, the catalyst was dried in a drying oven (Fanem model AH-T, Brazil) at 60°C for 12 hours. After impregnation of the last salt, the catalyst was dried at 60°C for 24 hours and calcined in air at 350°C for 5 hours (heating rate of 10°C between 60 and 350°C). The catalyst was analyzed for iron, copper, silica and potassium by fluorescence spectroscopy.
The calcined catalyst was reduced in situ in a mixture of H2 and CO in a molar proportion of 1:1 for 8 hours at 240°C and 20 atm.
B. Fischer-Tropsch Reaction
The FTS was carried out in a stirred, semi-batch operated 1 L autoclave reactor with catalyst being suspended in purified n-paraffin mixture of known composition (Synth, Brazil - melting point range between 60 and 62°C). The n-paraffin mixture is inert under the present reaction conditions.
The reaction was conducted with 10 grams of catalyst suspended in 300 grams of paraffin in a high-pressure autoclave reactor (Parr Instruments model 4571). Tests were conducted at total pressure ranging from 20 to 30 atm, 240 to 270°C, and a 1:1 H2:CO molar feed ratio. Synthesis gas was prepared by mixing H2 and CO via two mass flow controllers, enabling the desired H2/CO ratio to be obtained. The feed was introduced into the reactor below the agitator. The reaction was conducted under constant mechanical agitation (800 rpm), and the impeller used was a gas entrainment impeller which provides constant recirculation of the syngas into the slurry phase.
The tests followed a 22 experimental planning with central composite. Conditions for each run are listed in Table 1. Experiments were done in duplicate and the results were based on the mean values.
Table 1. Operating conditions of the experimental planning.
C. Data Analysis
The data at any set of process conditions were obtained during 6.5 hours mass balance periods when the liquid products were allowed to accumulated in the reactor.
The liquid-phase products were analyzed in a Shimadzu (Model QP5050) gas chromatograph with mass spectrum (CGMS) equipped with thermal conductivity (TCD) and flame-ionization (FID) detectors. Temperature programming (30°C to 300°C) with a packed OV-5 column (30 m x 0.25 mm ID x 0.25 μm film) allowed the identification of liquid-product Fischer-Tropsch hydrocarbons (C10 to C40). A microcomputer used to perform data acquisition and process control also controlled automatic chromatograph sampling.
III. RESULTS AND DISCUSSION
Iron catalysts form mostly straight-chain hydrocarbons. Fischer-Tropsch liquid products of particular value are diesel and kerosene (jet fuel), which range from about C10 to C25. Heavier waxy products (C25+) are of low value as final products but can be hydrocracked to lower molecular weight fuels such as gasoline, diesel and kerosene. As a specific chain-length is not possible to be produced, our aim is to study operating conditions that can produce diesel range hydrocarbons and heavy waxy products.
The liquid products obtained in all experiments consisted mainly of n-paraffins. The amount of olefins produced was negligible and was related to the mode of operation of the reactor which allows readsorption of olefins by the catalyst and its conversion to n-paraffins due to the high concentration of hydrogen in the reactor. The conversion of syngas into hydrocarbons was between 38 to 40% of the gas fed into the reactor, which were at the same conversion level reported in the literature (van Berge, 1997; Davis, 2003).
The distribution of the liquid products of the Fischer-Tropsch synthesis presents a bell shaped curve as shown in Fig. 1.

Figure 1. Distribution of the liquid product for run carried out at 240°C and 20 atm.
The effect of temperature and pressure over the liquid product distribution was evaluated using surface analysis methodology. Figures 2 to 5 show the mass fractions of four cuts of hydrocarbons as a function of temperature and pressure.
As shown in Figs. 2 and 3, the effect of temperature and pressure on the chain length is very complex. At low temperature (240°C) an increase in pressure leads to increasing mass fractions of low molecular weight hydrocarbons, thus favoring the production of lighter fractions rather than waxy products. Decreasing pressures favor an increase in liquid products of the range between C21 and C25 at high temperature (270°C).
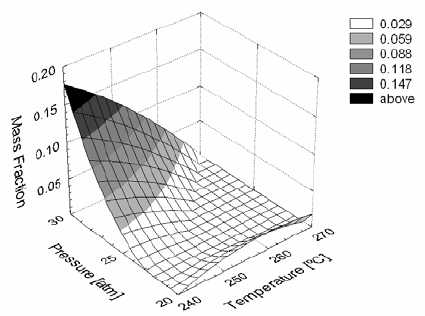
Figure 2. Mass fraction of hydrocarbons C20- obtained under different temperatures and pressures.

Figure 3. Mass fraction of hydrocarbons in the range of C23 and C25 obtained under different temperatures and pressures.
An increase in waxy hydrocarbon products was favored by mid-range pressures (between 24 and 27 atm) and was only slightly influenced by temperature (Fig. 4). However, increasing pressure and temperature have allowed the production of waxy products with more than 35 carbons in greater quantities (Fig. 5). Figure 5 also denotes that the temperature sensitivity for production of heavier hydrocarbons is at its peak at a pressure of about 27 atm, where the partial derivative with respect to the temperature of the response surface reaches its maximum.

Figure 4. Mass fraction of hydrocarbons in the range of C29 and C31 obtained under different temperatures and pressures.
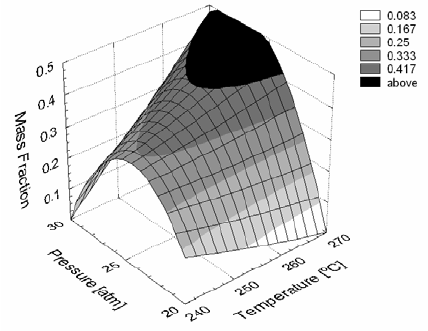
Figure 5. Mass fraction of hydrocarbons C31+ obtained under different temperatures and pressures.
The effect of pressure and temperature on the product distribution can be better examined analyzing the number average number of carbons. The number average number of carbons is a statistical index calculated dividing the first moment by the zeroth moment of the product distribution. This index represents the hydrocarbon chain length in which the mass fraction of the distribution is centered and can be used as a parameter to evaluate the polymerization degree of the Fischer-Tropsch reaction.
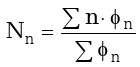
|
(2) |
The distribution can be further evaluated by the product distribution dispersion (or standard deviation) which is an index of how narrow or how broad the product distribution is. The dispersion can be calculated by:
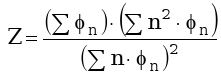
|
(3) |
Table 2 and Fig. 6 show the number average number of carbons for the reactions carried out with catalyst produced in this study. The results show that the catalyst has produced hydrocarbons with long chain length with a number average as high as 31 carbons. Analyzing the response surface, the maximum number average number of carbons would be obtained operating the reactor at 270°C and 27 atm. As may be inferred from Fig. 6, this maximum point is not an absolute one, and the surface behavior indicate that higher molecular weight products might be obtained by increasing the reaction temperature.
Table 2. Number average number of carbons and distribution dispersion.
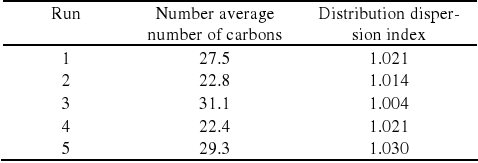

Figure 6. Number average number of carbons obtained under different temperatures and pressures.
The results for the product distribution dispersion index show that when the number average number of carbons is maximum, the dispersion index is relatively low, meaning that the product distribution is narrow. The value of 1.004 represents that the product distribution is within 5 carbons of distance from the number average number of carbons. For Run #3 presented in Table 2, this would mean that the liquid Fischer-Tropsch product consists mainly of hydrocarbon chains between C26 and C36, which was consistent with the experimental findings.
The other runs have presented dispersion index around 1.02, value that represents a broader product distribution where the distribution is within 8 carbons of distance from the number average number of carbons.
The experimental results did not show significant n-paraffins with more than 35 carbons which can be caused by space limitation inside the catalyst pores, which may also explain why the distribution dispersion index is much lower for the highest number average number of carbons.
This hypothesis is also supported by the skewness of the distribution (Eq. 4) which indicates if the distribution is well centered on the number average carbon number or if the distribution is shifted to the right or to the left.
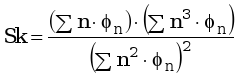
|
(4) |
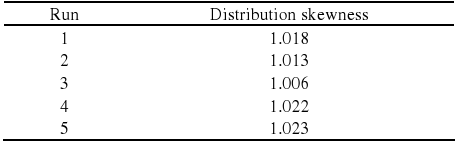
The results for the distribution skewness are presented in Table 3 and also show a value close to unit for the highest number average number of carbons indicating that the distribution is well centered. Other runs have presented skewness higher than 1.00 indicating a shift towards higher hydrocarbons chain lengths.
Table 3. Distribution skewness.
The ratio of mass of hydrocarbon per mass of carbon monoxide fed to the reactor is a valuable information that can be used for process optimization. The conversion of syngas into hydrocarbons did not change much with operating condition and was between 38 to 40% of the gas fed into the reactor. Operating at higher pressures resulted in the highest conversions into hydrocarbons. As such the concentration of gases in the reactor and the productivity increased with increasing operating pressures.
The mass of hydrocarbon per mass of carbon monoxide fed to the reactor is presented in Table 4. The results on mass of hydrocarbon per mass of carbon monoxide fed to the reactor confirm the results observed previously where at low temperature an increase in pressure favors the production of lighter products rather than waxy products. Decreasing pressures favor an increase in liquid products of the diesel range at high temperature (270°C).
The increase in waxes was favored by high pressures and high temperature, where C34+ hydrocarbons presented higher productivity.
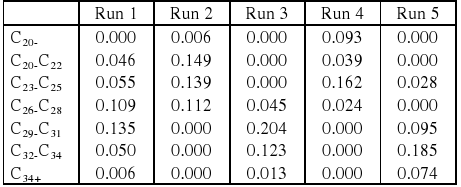
The increase in waxes was favored by high pressures and high temperature, where C34+ hydrocarbons presented higher productivity.
V. CONCLUSIONS
Fischer-Tropsch synthesis can be used to produce transportation fuels and olefins from natural gas and the polymerization conditions can be set to maximize the production of a certain product generated by the FTS reaction, such as diesel or heavy waxy products.
The process conditions (pressure and temperature) have a complex relationship with the liquid product distribution produced using iron catalyst. Surface responses showed that the system can be optimized toward lower number average number of carbons leading to higher selectivity of diesel cut or to higher selectivity of heavy waxy products condition which high pressure and temperature should be employed.
Analysis of the distribution dispersion and skewness indicated that the carbon monoxide polymerization degree may be limited by pore size.
Further research will be carried out to improve the catalyst, studying the effect of potassium content on the product distribution and reaction conversion.
NOMENCLATURE
n number of carbons
Nn number average number of carbons
Sk skewness
Z distribution dispersion 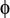 n mass fraction of the hydrocarbon with n number of carbons
n mass fraction of the hydrocarbon with n number of carbons
ACKNOWLEDGMENT
The authors gratefully acknowledge the financial support of the Brazilian research funding institution CNPq/CTPetro (Conselho Nacional de Desenvolvimento Científico), CAPES for the award of a scholarship, Prof. J.M.Sasaki and Laboratório de Raios-X for the X-Ray analysis and Prof. F.J.Q. Monte for the mass spectrum chromatographs.
REFERENCES
1. Davis, B.H. "Fischer-Tropsch Synthesis: Relationship between Iron Catalyst Composition and Process Variables". Catal. Today, 84, 83-98 (2003). [ Links ]
2. Donnelly, T.J. and C.N. Satterfield, "Product distributions of the Fischer-Tropsch synthesis on precipitated iron catalysts." Appl. Catal., 52, 93-114 (1989). [ Links ]
3. Eliason, S.A. and C.H. Bartholomew, "Reaction and Deactivation Kinetics for Fischer-Tropsch Synthesis on Unpromoted and Potassium-Promoted Iron Catalysts". Appl. Catal., 186, 229-243 (1999). [ Links ]
4. Fernandes, F.A.N., "Polymerization Kinetics of Fischer-Tropsch Reaction on Iron Based Catalysts and Product Grade Optimization". Chem. Eng. Tech., 28, 930-938 (2005). [ Links ]
5. Fernandes, F.A.N., "Modeling and product grade optimization of Fischer-Tropsch Synthesis in a slurry reactor". Ind. Eng. Chem. Res., 45, 1047-1057 (2006). [ Links ]
6. Fernandes, F.A.N. and E.M.M. Sousa, "Fischer-Tropsch Synthesis Product Grade Optimization in a Fluidized Bed Reactor". AIChE J., 2844-2850 (2006). [ Links ]
7. Huff, G.A. and C.N. Satterfield, "Evidence for two chain growth probabilities on iron catalysts in the Fischer-Tropsch synthesis". J. Catal., 85, 370-379 (1984). [ Links ]
8. Li, S., S. Krishnamoorthy, A. Li, G.D. Meitzner and E. Iglesia, "Promoted Iron-Based Catalysts for the Fischer-Tropsch Synthesis: Design, Synthesis, Site Densities, and Catalytic Properties." J. Catal., 206, 202-217 (2002). [ Links ]
9. Madon, R.J. and W.F. Taylor, "Fischer-Tropsch synthesis on a precipitated iron catalyst." J. Catal., 69, 32-43 (1981). [ Links ]
10. Maitlis, P.M., R. Quyoum, H.C. Long and M.L. Turner, "Towards a chemical understanding of the Fischer-Tropsch reaction: Alkene formation", Appl. Catal., 186, 363-374 (1999). [ Links ]
11. Patzlaff, J., Y. Liu, C. Graffmann and J. Gaube, "Studies on product distributions of iron and cobalt catalyzed Fischer-Tropsch synthesis." Appl. Catal., 186, 109-119 (1999). [ Links ]
12. Raje, A.P. and B.H. Davis, "Fischer-Tropsch Synthesis over Iron-Based Catalysts in a Slurry Reactor. Reaction Rates, Selectivities and Implications for Improving Hydrocarbon Productivity." Cat.Today, 36, 335-345 (1997). [ Links ]
13. van Berge, P.J., "Natural gas conversion IV". Studies Surf. Sci. Cat., 107, 207 (1997). [ Links ]
14. van der Laan, G.P. and A.A.C.M. Beenackers, "Hydrocarbon Selectivity Model for the Gas-Solid Fischer-Tropsch Synthesis on Precipitated Iron Catalysts". Ind. Eng. Chem. Res., 38, 1277-1290 (1999). [ Links ]
15. van Steen, E. and H. Schulz, "Polymerization Kinetics of the Fischer-Tropsch CO Hydrogenation using Iron and Cobalt Based Catalysts." Appl. Catal., 186, 309-320 (1999).
[ Links ]
Received: December 5, 2006
Accepted: April 3, 2007
Recommended by Subject Editor: Orlando Alfano














