Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Latin American applied research
Print version ISSN 0327-0793
Lat. Am. appl. res. vol.38 no.1 Bahía Blanca Jan. 2008
Determination of some physical and transport properties of palm oil and of its methyl esters
P. C. Narváez, S. M. Rincón, L. Z. Castañeda and F. J. Sánchez
Departamento de Ingeniería Química, Universidad Nacional de Colombia, Ciudad Universitaria, Edificio 412,
Bogotá D. C., Colombia. pcnarvaezr@unal.edu.co
Abstract — In this article the results of the experimental determination of a group of physical and transport properties of palm oil and of its methyl esters are presented, so as to generate reliable information for the execution of works done by chemical engineers, such as the modeling, the equipment sizing and the simulation of processes. Melting ranges, heats of fusion, boiling points, heats of combustion, were experimentally determined, and density, viscosity and heat capacity as a function of temperature were measured. The experimental values were adjusted to empirical models. In addition, density, viscosity and heat capacity as a function of temperature for three mixtures of palm oil and its methyl esters were measured, from where it was concluded that the density and heat capacity can be calculated as if the mixture would behave as ideal solutions, while the viscosity can be calculated with Andrade's model.
Keywords — Palm Oil; Methyl Esters; Biodiesel; Properties.
I. INTRODUCTION
The World's decrease of the probed reserves of oil and the constant conflict in the Middle East, have taken the oil price to historical levels, and this makes more feasible, from an economical point of view, to replace some of the petrochemical derivatives which make part of the every day life, for some other coming from renewable raw materials and more friendly with the environment. Within these raw materials, oils and fats can be transformed into a large quantity of products, with diverse applications and uses.
The possibility to use fatty acid methyl esters (FAME), product of the methanolysis of oil and fats, as a partial or total substitute of petrochemical diesel (Barnwal and Sharma, 2005), and as a raw material to produce fatty acid methyl esters sulphonates (MES), which can replace the traditional linear alkyl benzene sulphonates (LABS) in detergents (Hama and Ohbu. 2002), has increased the interest in developing processes that increase the productivity and facilitate the stages of separation and purification.
Colombia is the fifth World palm oil producing country and the first in Latin America, with an annual production of about 650,000 tons. The agricultural industry of palm oil is one of the main job-generating in the Colombian rural sector and an important protagonist in the export commerce of oils and fats. The Colombian government has identified this potential, and based on the legislation promotes the cultivation of African palm (Elaeis guineensis) and the production of biodiesel, which by the year 2008 will be added to the petrochemical diesel in order to replace the 5%, in a similar way to that used for the oxygenation of gasoline with ethanol.
In this study the results of the measurement of some physical and transport properties of palm oil, its methyl esters and three of its mixtures, are presented. Melting ranges, heats of fusion, boiling points and heats of combustion were experimentally determined, and the properties density, heat capacity and viscosity, were measured as a function of temperature.
With these experimental values and the empirical models obtained from them, it is pretended, for example, in the case of a process simulation, to facilitate the evaluation of the calculation methods of the properties, the validation of properties and the estimation of parameters not available in the data base of the simulator. To have reliable values of properties is fundamental in order for the results of the processes simulation and the posterior economical evaluation gets closer to reality (Carlson, 1996).
In the case of the density and viscosity of palm oil methyl esters, the study is justified because, although Tate et al. (2006a, 2006b) obtained densities and kinematics viscosities of FAME from 20°C to 300ºC, FAME from palm oil was not included. In other hand, even though researchers have reported some models to predict the viscosity of FAME from their fatty acid composition (Allen, 1999), or from the pure component topological index (Shu, 2007), these models did not relate viscosity and temperature.
II. METHODS
A. Material
Refined, bleached and deodorized palm oil edible grade (RBD) obtained from INTERGRASAS S. A. (Bogota D.C.). The origin was the eastern production zone of palm oil in Colombia, and the fatty acid composition, determined by GC, and some of its properties are shown in Table 1. Methanol, sodium hydroxide, pyridine, benzoic acid and hydrochloric acid were of analytical grade obtained from MERCK (Darmstadt, Germany). Palm oil methyl ester of >98.5% purity, free of glycerol, 0.15 wt% of water and acid value 0.13 mg KOH/g, was obtained through methanolysis of the palm oil, under the conditions described further. Reference standars chromatographically pure as methyl palmitate, methyl oleate, monopalmitin, dipalmitin, tripalmitin, triolein and the silylating agent N,O-bis (trimethylsilil)trifuoruacetamide (BSTFA), were purchased from Sigma Aldrich Chemical Company (St. Louis, MO.). The internal standard, tricaprin, was obtained from Fluka (Buchs, Switzerland).
Table 1. Fatty acid composition and characterization of palm oil used in this work.

Ssaponification number and acid value were determined with an automatic titrator Mettler Toledo DL 53 (Mettler Toledo GmbH, Schwerzenbach, Switzerland). Average molecular weight was calculated from saponification number.
B. Experimental Procedure
For the methanolysis of palm oil a cylindrical glass reactor of 750 mL, equipped with a stainless steel turbine type impeller, reflux condenser, thermocouple type K and sampling port, was used. The reactor jacket was connected to a JULABO F34 circulating bath (JULABO Labortechnik GmbH, Seelbach, Germany), capable of maintaining the temperature within ±0.1 °C. The rotational speed of the impeller was 400 rpm (±1 rpm), and for providing the mixing requirements a HEIDOLPH RZR 2021 mechanical stirred was used (Heidolph Instruments GmbH, Swchwabach, Germany).
The reactor was charged with 400 g of palm oil and it was heated at 60°C. A known quantity of sodium hydroxide was dissolved in methanol, and this solution was added to the reactor. The molar ratio of methanol to oil was 6 to 1 and the catalyst was 1 wt% sodium hydroxide. After 120 minutes, the reacting blend was taken to a decanting funnel of 1 L, where it stayed for at least 2 hours. The upper layer was neutralized with an stoichiometry quantity of HCl (5 wt%) and it was washed until the pH of the lower layer was 7. Finally, the upper layer was transferred to a flask and it was vacuum distilled to remove the remaining water and methanol. The palm oil methyl esters were analyzed by gases chromatography to determine the quantities of palm oil, di and monoglycerides, and methyl esters, using the method further described.
Mixtures of palm oil and its methyl esters of 500g of 25 wt%, 50 wt% and 75 wt% of methyl esters, were prepared weighting the corresponding quantities and keeping them to 50°C with agitation for 30 minutes.
C. Analysis
Gases Chromatography (GC)
A sample of about 20 mg was silylated at room temperature, adding BSTFA and pyridine. The analysis was performed in a Hewlett-Packard 5890 Series II gas chromatograph (Hewlett-Packard Co., Germany) equipped with a split/splitless injection port, flame ionization detector and the HP ChemStation software. A SUPELCO SGE HT-5 aluminum clad capillary column of 12mx0.53mmx0.15µm was used (SGE International Pty. Ltd. Victoria, Australia. N2 was used as carrier gas to a flow of 8 ml/min and 50:1 split radio. The injector's temperature was 350°C and that of the detector 390°C. After a stabilizing period of one minute at 140°C the oven's temperature was increased up to 380°C at 20°C/min. The final temperature was kept during 10 minutes.
Differential Scanning Calorimetry (DSC)
The heat capacities, melting ranges and heats of fusion were determined in a DSC 2910 TA Instruments (TA Instruments Co., New Castle, DE). The DSC was calibrated with aluminum oxide before determination. Samples of 5 to 10 mg were placed in a hermetic aluminum capsule, and an empty capsule was used as reference. The N2 source was adjusted to a flow of 20 mL/min. The capsules were heated at 10°C/min from -30°C up to 100°C. The amplitude was 1°C and the period 60 s.
Thermogravimetric Analysis (TGA)
The boiling points were measured in a TA Instruments Model 2050 thermobalance (TA Instruments Co., New Castle, DE), using a platinum capsule. Samples of 5 to 10 mg were at 10 °C/min from 20°C to 600°C, with N2 atmosphere to a flow of 20 mL/min.
Heat of Combustion
Heats of combustion where measured with a Parr Calorimeter Model 1241 (Parr Instruments Co., Moline, IL, USA), in accordance with the procedure established in the ASTM D-240.
Density
The density was calculated from the measurements of specific gravity carried out with a Fischer hydrometer with a range of 0.800 to 0.910 (Fischer Scientific, Rochester, NY), and following the procedure described in the ASTM D-1298. The temperature was adjusted with a JULABO F34 circulating bath (JULABO Labortechnik GmbH, Seelbach, Germany) and it was measured with an ERTCO ASTM 9C thermometer (Ever Ready Thermometer Co., Germany).
Viscosity
The viscosity of palm oil and of its methyl esters were calculated from the kinematics viscosity and density values. The kinematics viscosity was measured following the established procedures in the ASTM D-445, using a Cannon - Fenske Routine capillary viscometer (Cannon Instrument Co., State College, PA), and a Koelher model K 23376 bath (Koelher Instrument Co., Bohemia, NY) .
III. RESULTS AND DISCUSSION
A. Melting Ranges and Boiling Points
The results of the DSC of palm oil are shown in Fig. 1. Palm oil presented a fusion event which begins at -9.21°C, and ends at 46.10°C, in which two endothermic peaks were observed, at 3.99°C and 31.46°C. These peaks can be associated to the melting points of the triglycerides present in a greater proportion in the oil. The heat of fusion between 20.13°C and 46.36°C was 10.01 kJ/kg. Siew (2000) and Basiron (1996) presented DSC melting and crystallization thermograms with two peaks between -22°C and 45°C, and between -43°C and 42°C, respectively. It is quite clear that the two peaks observed in palm oil are due to the olein and stearin fractions. Kirschenbauer (1964) reported a melting range from 27°C to 50°C and Siew (Tsang, 2000), based on solid fat content determination by nuclear magnetic resonance, reported that at 45°C the RBD palm oil is melted.
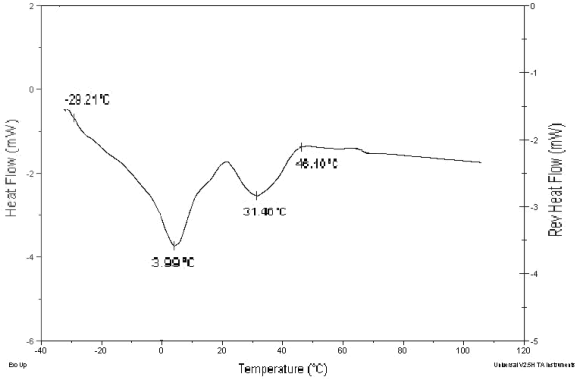
Figure 1. Diferencial Scanning Calorimetry of palm oil.
Palm oil methyl esters presented a fusion event which begins at -25.57°C, and finishes at 23.25°C. The associated heat in this event was 54.62 kJ/kg. Zhang (1994) reported melting points of 30.6°C and -19.8°C for methyl palmitate and methyl oleate, respectively, the same vales reported by Knothe and Dunn (2001), temperatures that are within the melting range measured in this work.
Figure 2 shows the thermogravimetric analysis of palm oil. The vaporization of palm oil took place at 369°C1. The vaporization of methyl esters took place at 180°C1. The values reported by Ma and Hanna (1999) for methyl oleate and methyl palmitate are 190.0°C and 196.0°C, respectively. The measured values in this work and those reported by Ma and Hanna (1999), are quite different from those reported by Goodrum (2002), who measured the boiling points of methyl esters of some fats and oils with values in the range of 338°C to 369°C.

Figure 2. Thermogravimetric analysis of palm oil
The lower limits of the measurement ranges of the properties of density, viscosity and heat capacity, were defined based on the DSC results. The measurement range for palm oil was 46°C <T<100°C, and for methyl ester 25°C < T<100°C.
B. Heats of Combustion
The heats of combustion are shown in Table 2. Srivastava and Prasad (2000) and Knothe and Dunn (2001), reported heats of combustion for vegetable oils in the range of 30 MJ/kg to 40 MJ/kg, relatively low compared to the petrochemical diesel (about 45 MJ/kg). The presence of oxygen linked in the molecule of triglyceride, reduces its heat of combustion. Kalam and Masjuki (2002) reported a value of 41.3 MJ/kg for palm oil methyl ester, and Knothe and Dunn (2001) reported values of 39.5 MJ/kg and 43.8 MJ/kg for methyl palmitate and methyl oleate, repectively.
Table 2. Heats of combustion (MJ/kg) of palm oil and of its methyl esters.

C. Density.
Palm oil and its methyl esters densities as a function of temperature are shown in Fig. 3. The Eq. 1 and 2 are the result of the lineal regression of the experimental data. The units of the Eq. are kg/m3 for density and °C for temperature.
| ρTG=-1.24T+945.14 | R2=0.997 | (1) |
| ρFAME=-1.18T+895.41 | R2=0.991 | (2) |
Alvarado (1997) reported some data for palm oil from Equator in the range of 30°C to 70°C, which fit to Eq. 3.
| ρTG=-0.70T+918 | R2=0.994 | (3) |
Within the common range for Eq. 1 and 3, the values differ in less than 1.2%. For Malaysian palm oil, Basiron (1996) reported an apparent density range from 888 to 889 kg/m3 for 215 samples at 50°C, and Siew (Tsang, 2000) a range from 889.6 to 891.0 kg/m3 for 244 samples at the same temperature, while the average value measured in this was 883.3 kg/m3, 0.8% lower than the mean of the reported ranges.
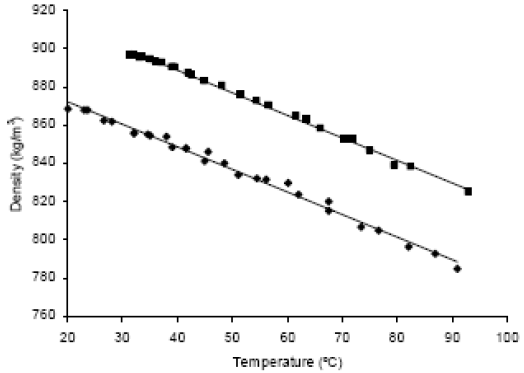
Figure 3. Densities of palm oil ( ) and of its methyl esters (
) and of its methyl esters ( â¦).
â¦).
In regard to methyl esters, Tate et al. (2006a) concluded, based on their experimental results, that density of FAME from canola, soybean and fish oils decrease linearly with temperature by 1.23 kg/m3°C, behavior which is similar to the found in this work, where density of palm oil methyl ester decrease linearly with temperature by 1.18 kg/m3°C. For esters obtained from palm oil, Kalam and Masjuki (2002) reported a typical value of 870 kg/m3 at 15°C, while Cheah et al. (1998) reported a range between 875 kg/m3 and 900 kg/m3 at the same temperature. Choo and Cheah (2000) reported a value of 870 kg/m3 at 23.6°C. In accordance with the results of DSC, 15°C and 23.6°C are within the melting range of methyl esters, and thus, this temperature makes no part of the range of Eq. 2. Nevertheless, and with the only object of conducting a comparison, the range was extended and values of 880 kg/m3 at 15°C, and 871 kg/m3 at 23.6°C were obtained. At 15°C the calculated value is inside the range reported by Cheah et al. (1998), and at 23.6°C the difference is less than 0.2% respect the value reported by Choo and Cheah (2000).
D. Viscosity
The viscosities of palm oil and of its methyl esters as a function of temperature are shown in Fig. 4, and Eq. 4 and 5 corresponds to the co-relation of the experimental data. The units of the Eq. are cP for viscosity and °C for temperature.
| LnμTG=1.59LnT+9.63 | R2=0.991 | (4) |
| LnμFAME=0.87LnT+4.68 | R2=0.995 | (5) |
Even though the function that expresses in a better form the relation of the viscosity and the temperature is Andrade's model (Krisnangkura et al., 2006), the data obtained in this work fits better to the function presented in Eq. 4 and 5. For palm oil, the reported data by Alvarado (1997) differs from the determined data in this work up to a 10%.
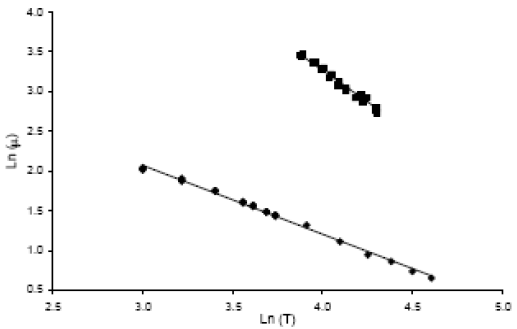
Figure 4. Viscosities of palm oil ( ) and of its methyl esters (â¦
) and of its methyl esters (â¦ ).
).
For methyl esters, Tate et al. (2006b), reported that kinematics viscosity of FAME from canola and soybean oils can be predicted by a modified Andrade's equation. Kalam and Masjuki (2002) reported a kinematics viscosity of 4.70 cSt at 40°C, Cheah et al. (1998) 4.44 cSt, and Shu et al. (2007) reported a viscosity of 3.59 cP at the same temperature. The measured viscosity at 40°C in this work was 5.13 cSt, 4.35 cP, greater than the maximum value approved for the European Union for biodiesel, but within the accepted range by the ASTM2.
The discrepancies between the measured and the reported values in this work can be attributed to the differences in the composition of the oils used, and to the presence of impurities in the FAME, principally di and monoglycerides. The viscosity of fat and oils greatly depends on the structure of the compound, and it is affected by factors such as chain length, the position, nature and number of double links and the nature of the oxygenated molecules (Knothe and Steidley, 2005). The longer the fatty acid hydrocarbon chain, the higher viscosity the FAME has; on the other hand, the greater the unsaturated bonds, the lower the viscosity of the FAME (Shu et al., 2007).
However, when the composition of palm oil used by Shu et al. (2007) was compared with the composition of palm oil used in this work; differences are too small to justify the discrepancies observed in methyl esters viscosities. Then, the discrepancies could be attributed to a higher content of di and monoglycerides (about 1.5 wt% in this case), which viscosities are higher than methyl ester. Moreover, di and monoglycerides are emulsifying agents and its presence in a mixture, still in low concentrations, could increase the viscosity.
E. Heat Capacity
The heat capacity of palm oil and of its methyl esters were presented in Fig. 5, and Eq. 6 and 7 are the result of the correlation of experimental data. The equation units are J/g°C for the heat capacity °C for the temperature.
| cP TG=0.0059T+1.617 | R2=0.993 | (6) |
| cP FAME=0.0050T+0.987 | R2=0.988 | (7) |
Alvarado (1997) reported a constant value of 2.20 J/ g°C for crude palm oil, in the range of 30°C to 70°C.
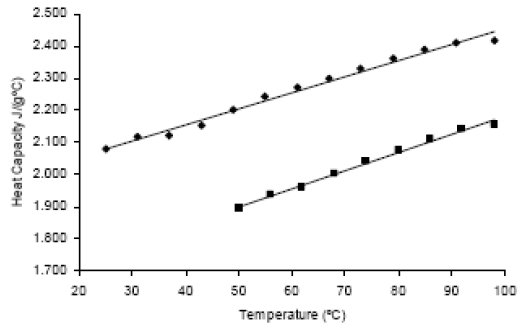
Figure 5. Heat Capacities of palm oil ( ) and of its methyl esters (â¦
) and of its methyl esters (â¦ ).
).
F. Mixture properties
Figures 6 to 8 show the behavior of the density, viscosity and heat capacity, as a function of temperature, for mixtures of palm oil and its methyl esters. For the density and the heat capacity the behavior adjusts to the calculation model of properties for ideal solutions. This means that the property values can be calculated with Eq. 8 and 9, with deviations of less than 1% with respect to the experimental value of the density, and less than 2% with respect to the experimental value of the heat capacity.
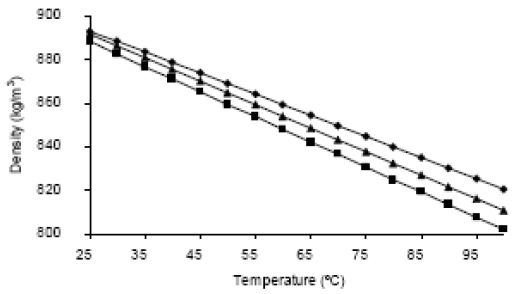
Figure 6. Densities of mixtures of palm oil and its methyl esters (â¦ ) 25 % (
) 25 % ( ) 50% (
) 50% ( ) 75% in weight of methyl esters.
) 75% in weight of methyl esters.
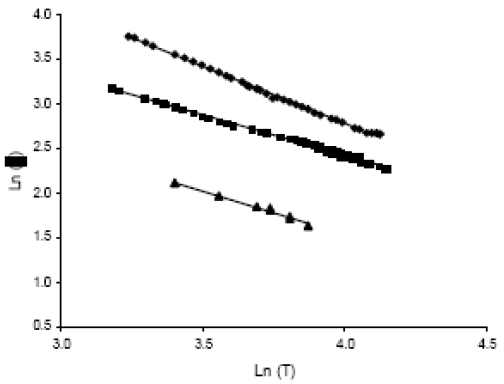
Figure 7. Viscosities of mixtures of palm oil and its methyl esters (â¦ ) 25 % (
) 25 % ( ) 50% (
) 50% ( ) 75% in weight of methyl esters.
) 75% in weight of methyl esters.

Fig. 8. Heat capacities of mixtures of palm oil and its methyl esters (â¦ ) 25 % (
) 25 % ( ) 50% (
) 50% ( ) 75% in weight of methyl esters.
) 75% in weight of methyl esters.
This behavior agrees with the reported by Goodrum and Eiteman (1996), who demonstrated that the binary mixtures of triglycerides behave as ideal solutions for the density and heat capacity. In these equations, x is the molar fraction.
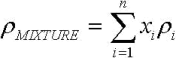
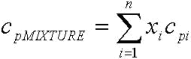
Regarding viscosity, the experimental values of mixtures fits accurately to Andrade´s model, which is shown on Eq. 10. The units are cP for viscosity and K for temperature.
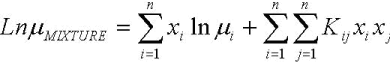 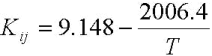 | (10) |
The average difference between the experimental and calculated values is of 10%. Goodrum and Eiteman (1996), demonstrated that the viscosity of triglycerides mixtures can be calculated using the empirical models of Kendall and Monroe, Eq. 11, while Krisnangkura et al. (2006), established that for a liquid mixture non associated, as in this case, the viscosity can be calculated using Eq. 12.
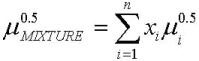 | (11) |
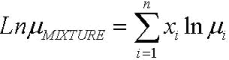 | (12) |
FAME mixtures are non associated liquids and due to the similarity, the components do not interact with each other and thus should behave in a similar manner as an individual component. Allen et al. (1999) reported that the Grunberg-Nissan equation, Eq. 13, is the most suitable for computing viscosity of liquid mixtures. In this ecuation, Gij is the interaction parameter that could be neglected in the case of FAME. In Eq. 10, although Kij is an interaction parameter, it includes the temperature dependency, and then can not be neglected.
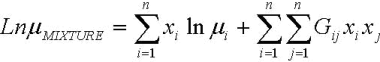 | (13) |
III. CONCLUSIONS
Melting ranges, boiling points and combustion heats of palm oil and of its methyl esters were measured, experimental values of the density, viscosity, and heat capacity of palm oil, of its methyl esters and of some mixtures of them were determined, as a function of temperature. In the studied range, the density and the heat capacity of palm oil and of its methyl esters adjust to a lineal function of temperature, while the viscosity adjusts to a lineal function of temperature natural logarithm. The density and heat capacity of the mixtures can be calculated from the properties of palm oil and of its methyl esters, using the calculation model of properties for ideal solutions. Andrade's model allows calculating the viscosity of mixtures with deviations lower than 10% with regard to the experimental data.
NOMENCLATURE
| cp | Heat capacity |
| G | Binary coefficient for the calculation of the viscosity in Grunberg-Nissan equation |
| K | Binary coefficient for the calculation of the viscosity in Andrade's model. |
| T | Temperature |
| i,j | Sub-index to indicate the component of a mixture |
| n | Number of components of a mixture. |
| x | Molar fraction |
| μ | Viscosity |
| ρ | Density |
| FAME | Palm oil methyl esters |
| TG | Palm oil |
1 The measured temperatures were corrected at a barometrical pressure of 760 mmHg using Sidney Young Eq.
2 The Standards EN 14214 and DIN V 51606, applicable in Europe, established a range for the kinematics viscosity at 40°C for the biodiesel from 3.5 to 5.0 cSt, while the ASTM D6751, applicable in the United States, established a range from 1.9 to 6.0 cSt.
AKNOWLEDGMENTS
This work is part of the project "Ésteres surfactantes derivados del aceite de palma", sponsored by COLCIENCIAS (Contract RC 389) and Universidad Nacional de Colombia.
REFERENCES
1. Allen, C.A.W., K.C. Watts, R.G. Ackman and M.J. Pegg, "Predicting the viscosity of biodiesel fuels from their fatty acid ester composition", Fuel, 78, 1319-1326 (1999). [ Links ]
2. Alvarado, J., "Propiedades físicas de aceites de pulpa y de almendra de palma africana a diferentes temperatures", Proc. Generalidades de la palma africana, Santo Domingo de los Colorados, Octubre (1997). [ Links ]
3. Barnwal, B.K. and M.P. Sharma, "Prospects of biodiesel production from vegetable oils in India", Renew Sustain. Energy Rev., 9, 363-378 (2005). [ Links ]
4. Basiron, Y., "Palm Oil" in Bailey's Industrial Oil and Fat Products, 2, Edited by Hui Y.H., John Wiley & Sons, USA (1996). [ Links ]
5. Carlson, E., "Don't gamble with physical properties for simulations", Chem. Eng. Prog., 92, 35-46 (1996). [ Links ]
6. Chea, K.Y., Y.M. Choo, A.N. Ma and Y. Basiron, "Production Technology of Palm Diesel", Proc. of 1998 PORIM International Biofuel and Lubricant Conference, 207-226 (1998). [ Links ]
7. Choo, Y.M. and K.Y. Cheah, "Biofuel" Advances in Oil Palm Research, Ed. by Y. Basiron, B. Jalani and K. Chan, II, Malaysia Palm Oil Board, Malaysia (2000). [ Links ]
8. Goodrum. Y.W. and M.A. Eiteman, "Physical properties of low molecular weight triglycerides for the development of biodiesel fuel models", Bioresour. Biotechnol., 56, 55-60 (1996). [ Links ]
9. Goodrum, J.W., "Volatility and boiling points of biodiesel from vegetable oils and tallow", Biomass Bioenergy, 22, 205-211 (2002). [ Links ]
10. Hama, I., and K. Ohbu, "New technology and industries on the use of palm oil in oleochemical industries", 2002 International Oil Palm Conference, Nusa Dua, Bali, 1-14 (2002). [ Links ]
11. Kalam, M.A. and H.H. Masjuki, "Biodiesel from palmoil- an analysis of its properties and potential" Biomass Bioenergy, 23, 471-479 (2002). [ Links ]
12. Kirschenbauer, H., Fats an Oils, Second Edition, Reinhold Publisching Corporation, New York (1964). [ Links ]
13. Knothe, G. and R. Dunn, "Biofuels derived from vegetable oils and fats", Oleochemical Manufacture and Applications, Edited by F. Gunstone and R. Hamilton, Sheffield Academic Press, England (2001). [ Links ]
14. Knothe, G. and K. Steidley, "Kinematic viscosity of biodiesel fuel components and related compounds. Influence of compound structure and comparison to petrodiesel fuel components", Fuel, 84, 1059-1065 (2005). [ Links ]
15. Krisnangkura, K., T. Yimsuwan and R. Pairintra, "An empirical approach in predicting biodiesel viscosity at various temperatures", Fuel, 85, 107-113 (2006). [ Links ]
16. Ma, F. and M.A. Hanna, "Biodiesel production: a review" Bioresour. Technol., 70, 1-15 (1999). [ Links ]
17. Shu, Q., B. Yang, J. Yang and S. Qing, "Predicting the viscosity of biodiesel fuels based on the mixture topological index method", Fuel, 86, 1849-1854 (2007). [ Links ]
18. Siew, W., "Analysis of palm and palm kernel oils" in Advances in Oil Palm Research, Ed. by Y. Basiron, B. Jalani and K. Chan, II, Malaysia Palm Oil Board, Malaysia (2000). [ Links ]
19. Srivastava, A. and R. Prasad, "Triglycerides-based diesel fuels", Renew. Sustain. Energy Rev., 4, 111-133 (2000). [ Links ]
20. Tang, T.S., "Composition and Properties of palm Oil Products", in Advances in Oil Palm Research, Ed. by Y. Basiron, B. Jalani and K. Chan, II, Malaysia Palm Oil Board, Malaysia (2000). [ Links ]
21. Tate, R.E., K.C. Watts, C.A. Allen and K.I. Wilkie, "The densities of three biodiesel fuels at temperatures up to 300ºC", Fuel, 85, 1004-1009 (2006a). [ Links ]
22. Tate, R.E., K.C. Watts, C.A. Allen and K.I. Wilkie, "The viscosities of three biodiesel fuels at temperatures up to 300ºC", Fuel, 85, 1010-1015 (2006b). [ Links ]
23. Zhang, D., Crystallization Characteristics and Fuel Properties of Tallow Methyl Esters, Master Thesis, Food Science and Technology, University of Nebraska - Lincoln (1994). [ Links ]
Received: February 9, 2007.
Accepted: April 15, 2007.
Recommended by Subject Editor Ricardo Gómez.














