Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Latin American applied research
versión impresa ISSN 0327-0793
Lat. Am. appl. res. v.38 n.1 Bahía Blanca ene. 2008
Preparation of palladium-silver alloy membranes for hydrogen permeation
E. L. Foletto*, J. V. Wirbitzki Da Silveira and S. L. Jahn
Chemical Engineering Department, Federal Universtiy of Santa Maria, 97150-900, Santa Maria,RS, Brazil
* e-mail: foletto@smail.ufsm.br
Abstract — Pd-Ag alloy membranes (with different silver contents) have been prepared by electroless plating technique for hydrogen separation. The surface morphology and phase structures of the composite membranes were investigated using a scanning electron microscope and a X-ray diffractometer, respectively. Hydrogen permeation experiments through the membranes synthesized at different hydrogen pressures (50 - 250 kPa) and temperatures (623 - 823 K) are reported. The experimental results reveal that the membranes showed a considerable enhancement in the hydrogen permeation and that the contribution of surface process for permeation was significant at high temperatures.
Keywords — Membrane Preparation; Palladium-Silver; Hydrogen Permeation.
I. INTRODUCTION
Hydrogen selective membranes are important for technological process such as separation and recovery of hydrogen from process steam reforming, purification of hydrogen for fuel cell application and chemical reactions in membrane reactors (Chai et al., 1995; Kikuchi, 1995; A.-Petersen et al., 1998; Wieland et al., 2002; Kusakabe et al., 2005). Palladium membranes have been used in hydrogen separation because present high selectivity (Tosti et al., 2003; Checchetto et al., 2004). However, they present problem of hydrogen embrittlement, a phenomenon in which dissolved hydrogen tend to cause different elongation of metallic film on glass support, leading it to fracture after repeated pressure and thermal cycling (Tosti et al., 2001). In order to avoid the embrittlement, alloying of Pd with group IB metals such a silver is generally made. (Fort et al., 1975). Another advantage of alloying is that the mechanical strength can be higher than for pure palladium. In general, a Pd-membrane becomes brittle after certain cycles of α-β hydride transformations due to the accompanied lattice expansion. In, for example, palladium-silver alloys, the lattice has already been expanded by the silver atoms, and the PdAg lattice is less influenced by hydrogen and thus less brittle than the pure Pd lattice (Amandusson et al., 2001). A optimal value of the hydrogen permeation rate is reached for a silver content around 25 wt. % Ag (Knapton, 1977; Uemiya et al., 1991). Zhang et al. (2003) had observed that at 473 K or less, the hydrogen permeability of Pd-Ag alloy membranes increased with the Ag content until 30 wt. %. Other alloys like cerium-palladium, palladium-tantalum, palladium-niobium, yttrium-palladium and cupper-palladium showed high permeability and mechanical strength (Fort et al., 1975; Buxbaum and Kinney, 1996; Roa et al., 2003; Siriwardane et al., 2003; Howard et al., 2004; Tong et al., 2006). There are various methods reported in the literature for fabrication of composite Pd-based membrane for hydrogen permeation such as chemical vapor deposition (Xomeritakis and Lin, 1998; Jun and Lee, 2000; Itoh et al., 2005), sputter deposition (Jayaraman et al., 1995; Jayaraman and Lin, 1995; McCool, et al., 1999), electroless plating with osmosis (Souleimanova et al., 2001; Souleimanova et al., 2002) and electroless plating (Tong et al., 2005; Lin and Chang, 2005; Tanaka et al., 2005; Rahimi and Iraji, 2005), being this last more used for having as advantages low cost and the use of very simple equipment (Altinisik et al., 2005).
The goal of this work was to prepare palladium-based membranes with different silver contents, in order to investigate the influence of Ag concentration on hydrogen permeation. Characterization studies of Pd-Ag alloy membranes were performed using SEM and XRD.
II. METHODS
Porous Vycor glass tube (Corning Glass, Inc.) was employed as support for the manufacturing of Pd-Ag alloy membranes. The specifications of the support are: length of 10 cm, outer diameter of 10 mm, inner diameter of 7 mm and average pore size of 50 Ǻ. The porous tubes were first cleaned with hydrogen peroxide, trichloroethylene and deionized water as procedure described by Cheng and Yeung (1999). Following the cleaning, a procedure with palladium predeposition was performed before co-deposition of Pd-Ag by method eletroless plating. Initially, the support was seeded with Pd crystallites that formed nucleation sites for subsequent plating of a Pd-Ag film on the substrate. This process involved immersion of the substrate in SnCl2.2H2O 5mM solution for 5 min followed by another 5 min in PdCl2 5mM solution. This sequence was repeated 9 times. Afterwards the palladium and silver deposition was carried out at 323 K, for 60 min in a plating bath containing a 70 mL solution with composition listed in Table 1. After the plating the membrane was washed with deionized water and allowed to air dry. The total amount of metals in plating solution was kept constant at 0,911 mM, however the content of palladium and silver was altered in order to form a metallic film on the membranes with different silver contents. After the plating, the samples were sintered at 823 K in a pure hydrogen atmosphere for 3 h to promote alloying.
Table 1. Composition of the electroless plating solution.

The morphology of metallic film on membrane was analyzed by the scanning electron microscope (SEM) (Joel 2000FX) and the phase structures of the film were examined by a X-ray diffractometer (XRD) (Shimatzu, with a CuKα radiation). The thickness of the films was measured from the SEM micrographs. The surface compositions were analysed with an energy dispersive spectrometer (EDS, Joel).
Permeation experiments were carried out using pure hydrogen in a conventional gas permeation apparatus. The membranes were loaded into a stainless steel shell and centered in a tube furnace. For membrane sealing, graphite rings were used. The measurements were made in a temperature range between 623 and 823 K and pressure differences up to 250 kPa. The total area of the composite sample exposed to the gas was 20 cm2. The volumetric flow rate of permeated gas was measured at ambient temperature and atmospheric pressure with a soap-bubble flow meter after steady state (approximately 1 h) had been achieved at the desired temperature.
The hydrogen permeation through palladium-based membranes was described by Eq. (1) as follows (Dittmeyer et al., 2001; Huang et al., 2003):
J = β (Phn - Pl n ) (1)
where J is the gas flux, β is temperature dependent constant, n is a constant power of the pressure, Ph - Pl are pressure on feed and permeate sides, respectively.
The measured hydrogen fluxes through palladium-based membranes at different temperatures served to estimate the activation energy as described by a Arrhenius law as follows:
β = βo exp(-EA/RT) (2)
where EA is the activation apparent energy, βo is the Arrhenius constant, R is the gas constant and T is the temperature.
III. RESULTS
Membranes prepared under different conditions exhibited differences in composition and thickness (see Table 2).
Table 2. Composition and thickness of membranes.

Table 2 depict the microstructures of membranes with silver content of 5, 24 and 35 wt.% Ag prepared from plating solutions with 5, 20 and 30 wt.% Ag, respectively.
Figure 1 and Fig. 2 shows the XRD patterns of M3 and M4 samples (before and after thermal treatment), respectively. It is observed that deposited Pd and Ag are in separate crystalline phases, before thermal treatment. After treatment in a hydrogen atmosphere at 823 K, the samples become Pd-Ag alloy crystallized. The intensity of silver diffraction peak decreased with the thermal treatment to promote Pd-Ag alloying.
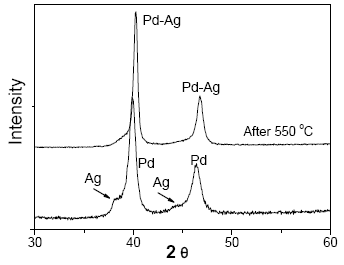
Fig. 1. XRD pattern of M3 membrane.
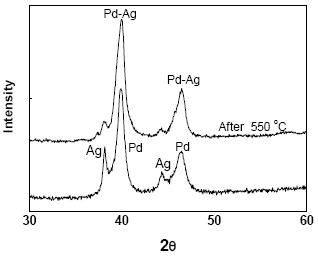
Fig. 2. XRD pattern of M4 membrane.
The surface morphology of M1, M2, M3 and M4 membranes is shown in Fig. 3, Fig. 4, Fig. 5 and Fig. 6, respectively. Figure 3 reveals that the grain growth of M1 sample was not uniform with some of the grains growing more rapidly than others, resulting in a metallic film with nonuniform grain size. The membranes containing silver had smaller grains than the pure Pd membrane, due to the preferential silver deposition

Fig. 3. Scanning electron micrograph of pure Pd membrane (M1 sample).

Fig. 4. Scanning electron micrograph of M2 membrane.
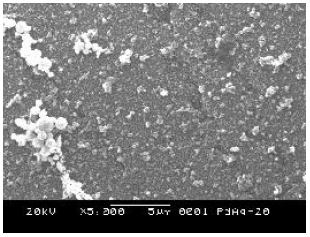
Fig. 5. Scanning electron micrograph of M3 membrane.

Fig. 6. Scanning electron micrograph of M4 membrane.
Comparing the microstructure of M2 (Fig. 4), M3 (Fig. 5) and M4 (Fig. 6) samples, it can be seen that the increase of silver content in the metallic film lead to finer grains and more uniform microstructures. Cheng and Yeung (1999) showed that Pd film microstructure can be significantly altered through addition of different silver contents in the electroless plating bath.
Tests of hydrogen permeation had only been carried with the M3 and M4 membranes . The M1 and M2 membranes presented problems during the tests, the metallic alloys peeled off the support. Some researchers had reported that at least 20 wt. % Ag is needed to prevent hydrogen embrittlement above ambient temperature (Fort et al., 1975; Grashoff et al., 1983; Paglieri and Way, 2002).
Figure 7 and Fig. 8 have shown the hydrogen permeation rate as a function of pressure difference at various temperatures, for M3 and M4 membranes, respectively. It can see that the values of n (determined by regression) of 0.57 and 0.67 shows that J is linear to (Phn - Pl n ). It indicate that the hydrogen permeation through the membranes deviated from Sievert´s law (n = 0.5). Value of 0.5 implicate that the diffusion through the membrane is rate limiting (Holleck, 1970). The values detected in these work indicate that the surface processes become rate limiting. Amandusson et al. (2001) and Jayaraman and Lin (1995) have found values of pressure power between 0.5 and 1, explaining that the surface process becoming rate determining step. Amandusson et al. (2001) conclude that for temperatures below 473 K, the bulk diffusion through the membranes is rate limiting, while at temperatures above 473 K, surface processes become rate limiting. Values of n between 0.5 and 1 have been reported for various types of palladium composite membranes (Dittmeyer et al., 2001).

Fig. 7. Hydrogen permeation rate though the M3 membrane as a function of pressure difference to the 0.57 exponent power, at various temperatures.

Fig. 8. Hydrogen permeation rate though the M4 membrane as a function of pressure difference to the 0.67 exponent power, at various temperatures.
It is also observed through the Fig. 7 and Fig. 8 that the hydrogen flux increases with rising temperature and pressure difference for both membranes. The results had shown that the M4 membrane showed a slightly lower permeability than the M3 membrane. Zhang et al. (2006) had observed that the hydrogen permeability of the PdAg alloy membranes tended to decrease with reduction of membrane thickness. It indicated that the difference between the overall hydrogen permeation to the thick and thin composite membranes could not be simply ascribed to interface effects. The hydrogen permeation is a complex function of many parameters, such as, hydrogen pressure, temperature, surface composition, bulk composition and also the composition gradient between surface and bulk, and likely also the surface morphology, as reported by Amandusson et al. (2001). The authors explained that hydrogen permeation at temperature above 473 K can be influenced not only by surface concentration but as too for a sharp silver concentration gradient. To determine the role of each parameter on its own is unfortunately not possible in this study. Uemiya et al. (1991) and Knapton (1977) showed that the hydrogen permeation rate of 26 wt.% Ag membrane is lower than the 23 wt.% Ag alloys. The optimal composition for maximal hydrogen permeation rate has often been claimed around 23-25 wt. % (Knapton, 1977; Dittmeyer et al., 2001; Amandusson et al., 2001).
Figure 9 shows the Arrhenius plots for determination of activation apparent energy for both the membranes. The values were find to be approximately 13.63 and 14.30 kJ/mol for the M3 and M4 membranes, respectively, and are a good agreement with literature data, which indicates a range of 10-22 kJ/mol (Holleck, 1970; Shu et al., 1991; Weyten et al., 2000; Li et al., 2000; Souleimanova et al., 2002).

Fig. 9. Arrhenius plots of hydrogen permeation for M3 and M4 membranes.
IV. CONCLUSIONS
Pd-Ag alloy membranes were synthesized by eletroless plating technique and tested for hydrogen permeation on different temperatures (623 - 823 K) and pressures (50 - 250 kPa). On temperatures above 623 K, silver content is an important parameter that influences hydrogen permeation through the membranes. Other important parameters are pressure, temperature and probably surface morphology. The membrane with higher silver content (M4) presented a lower hydrogen permeation rate than the membrane M3, and we suggest that this occurred probably because the first membrane is formed by a metallic alloy with lower grain size comparing to the second, and it would cause a higher hydrogen diffusion resistance through membrane. Under the conditions tested in this work, the surface reactions become rate limiting.
ACKNOWLEDGMENTS
The authors acknowledge the financial support from CNPq towards the project.
REFERENCES
1. A.-Petersen, K.; C.S. Nielsen and S.L. Jorgensen, "Membrane reforming for hydrogen", Catalysis Today, 46, 193-201 (1998). [ Links ]
2. Altinisik, O., M. Dogan and G. Dogu, "Preparation and characterization of palladium-plated porous glass for hydrogen enrichment", Catalysis Today, 105, 641-646 (2005). [ Links ]
3. Amandusson, H., L.G. Ekedahl and H. Dannetun, "Hydrogen permeation through surface modified Pd and PdAg membranes", Journal of Membrane Science, 193, 35-47 (2001). [ Links ]
4. Buxbaum, R.E. and A.B. Kinney, "Hydrogen transport through tubular membranes of palladium-coated tantalum and niobium", Ind. Eng. Chem. Res., 35, 530-537 (1996). [ Links ]
5. Chai, M., M. Machida, K. Eguchi and H. Arai, "Promotion of hydrogen permeation on metal-dispersed alumina membranes and its application to a membrane reactor for methane steam reforming", Applied Catalysis A: General, 110, 2, 239-250 (1995). [ Links ]
6. Checchetto, R., N. Bazzanella, B. Patton and A. Miotello, "Palladium membranes prepared by r.f. magnetron sputtering for hydrogen purification", Surface and Coatings Technology, 177, 73-79 (2004). [ Links ]
7. Cheng, Y.S. and K.L. Yeung, "Palladium-silver composite membranes by electroless plating technique", Journal of Membrane Science, 158, 127-141 (1999). [ Links ]
8. Dittmeyer, R., V. Höllein and K. Daub, "Membrane reactors for hydrogenation and dehydrogenation processes based on supported palladium, Journal of Molecular Catalysis A: Chemical, 173, 135-184 (2001). [ Links ]
9. Fort, D., J.P.G. Farr and I.R. Harris, "A comparison of palladium silver and palladium yttrium alloys as hydrogen separation membranes", J. Less Common Met., 39, 293-308 (1975). [ Links ]
10. Grashoff, G.J., C.E. Pilkington and C.W. Corti, "The purification of hydrogen, a review of the technology emphasizing the current status of palladium membrane diffusion", Platinum Met. Rev., 27, 157-169 (1983). [ Links ]
11. Holleck, G.L., "Diffusion and solubility of hydrogen in palladium and palladium-silver alloys", J. Phys. Chem., 74, 503-511 (1970). [ Links ]
12. Howard, B.H., R.P. Killmeyer, K.S. Rothenberger, A.V. Cugini, B.D. Morreale, R.M. Enick and F. Bustamante, "Hydrogen permeance of palladium-copper alloy membranes over a wide range of temperatures and pressures", Journal of Membrane Science, 241, 207-218 (2004). [ Links ]
13. Huang, T.-C., M.-C. Wei and H.-I. Chen, "Preparation of hydrogen-permselective palladium-silver alloy composite membranes by electroless co-deposition", Separation and Purification Technology, 32, 239-245 (2003). [ Links ]
14. Itoh, N., T. Akiha and T. Sato, "Preparation of thin palladium composite membrane tube by a CVD technique and its hydrogen permselectivity", Catalysis Today, 104, 231-237 (2005). [ Links ]
15. Jayaraman, V. and Y.S. Lin, "Synthesis and hydrogen permeation properties of ultrathin palladium-silver alloy membranes", Journal of Membrane Science, 104, 251-262 (1995). [ Links ]
16. Jayaraman, V.; Y.S. Lin, M. Pakala and R.Y. Lin, "Fabrication of ultrathin metallic membranes on ceramic supports by sputter deposition", Journal of Membrane Science, 99, 89-100 (1995). [ Links ]
17. Jun, C.-S. and K.-H. Lee, "Palladium and palladium alloy composite membranes prepared by metal-organic chemical vapor deposition method (cold-wall)", Journal of Membrane Science, 176, 121-130 (2000). [ Links ]
18. Kikuchi, E., "Palladium/ceramic membranes for selective hydrogen permeation and their application to membrane reactor", Catalysis Today, 25, 333-337 (1995). [ Links ]
19. Knapton, A.G., "Palladium alloys for hydrogen diffusion membranes", Platinum Met. Rev., 21, 44-50 (1977). [ Links ]
20. Kusakabe, K., S. Fumio, T. Eda, M. Oda and K.-I. Sotowa, "Hydrogen production in zirconia membrane reactors for use in PEM fuel cells", International Journal of Hydrogen Energy, 30, 989-994 (2005). [ Links ]
21. Li, A., W. Liang and R. Hughes, "The effect of carbon monoxide and steam on the hydrogen permeability of a Pd/stainless steel membrane", J. Membr. Sci., 165, 135-141 (2000). [ Links ]
22. Lin, W.-H. and H.-F. Chang, "Characterizations of Pd-Ag membrane prepared by sequential electroless deposition", Surface and Coatings Technology, 194, 157-166 (2005). [ Links ]
23. McCool, B., G. Xomeritakis and Y.S. Lin, "Composition control and hydrogen permeation characteristics of sputter deposited palladium-silver membranes", Journal of Membrane Science, 161, 67-76 (1999). [ Links ]
24. Paglieri, S. and J.D. Way, "Innovations in palladium membrane research", Sep. Purif. Meth., 31, 1-169 (2002). [ Links ]
25. Rahimi, F. and A. Iraji, "Effective factors on Pd growth on porous silicon by electroless-plating: response to hydrogen", Sensors and Actuators B: Chemical, 115, 164-169 (2005). [ Links ]
26. Roa, F., J.D. Way and S.N. Paglieri, "Process for preparing palladium alloy composite membranes for use in hydrogen separation, palladium alloy composite membranes and products incorporating or made from the membranes", United States Patent, N. 20030190486 (2003). [ Links ]
27. Shu, J., B.P.A. Grandjean, A. Van Neste and S. Kaliaguine, "Catalytic palladium-based membrane reactors: A Review", Can. J. Chem. Eng., 69, 1036-1060 (1991). [ Links ]
28. Siriwardane, R.V., J.A. Poston Jr., E.P. Fisher, T.H. Lee, S.E. Dorris and U. Balachandran, "Characterization of ceramic-metal composite hydrogen separation membranes consisting of barium oxide, cerium oxide, yttrium oxide and palladium", Applied Surface Science, 217, 43-49 (2003). [ Links ]
29. Souleimanova, R.S., A.S. Mukasyan and A. Varma, "Pd-composite membranes prepared by electroless plating and osmosis: synthesis, characterization and properties", Separation and Purification Technology, 25, 79-86 (2001). [ Links ]
30. Souleimanova, R.S., A.S. Mukasyan and A. Varma, "Pd Membranes formed by electroless plating with osmosis: h2 permeation studies", AIChE Journal, 48, 262-268 (2002). [ Links ]
31. Tanaka, D.A.P.; M.A. Tanco, S.-I. Niwa, Y. Wakui, F. Mizukami, T. Namba and T.M. Suzuki, "Preparation of palladium and silver alloy membrane on a porous α-alumina tube via simultaneous electroless plating", Journal of Membrane Science 247, 21-27 (2005). [ Links ]
32. Tong, J., Y. Matsumura, H. Suda and K. Haraya, "Thin and dense Pd/CeO2/MPSS composite membrane for hydrogen separation and steam reforming of methane", Separation and Purification Technology, 46, 1-10 (2005). [ Links ]
33. Tong, J., C. Su, K. Kuraoka, H. Suda and Y. Matsumura, "Preparation of thin Pd membrane on CeO2-modified porous metal by a combined method of electroless plating and chemical vapor deposition", Journal of Membrane Science, 269, 101-108, (2006). [ Links ]
34. Tosti, S., L. Bettinali, S. Castelli, F. Sarto, S. Scaglione, and V. Violante, "Sputtered, electroless, and rolled palladium-ceramic membranes", J. Membrane Sci., 196, 241-249 (2001). [ Links ]
35. Tosti, S., A. Adrover, A. Basile, V. Camilli, G. Chiappetta and V. Violante, "Characterization of thin wall Pd-Ag rolled membranes", International Journal of Hydrogen Energy, 28, 1, 105-112 (2003). [ Links ]
36. Uemiya, S., T. Matsuda and E. Kikuchi, "Hydrogen permeable palladium-alloy membrane supported on porous ceramics", J. Membr. Sci., 56, 315-325 (1991). [ Links ]
37. Weyten, H., J. Luyten, K. Keizer, L. Willems and R. Leysen, "Membrane performance: the key issues for dehydrogenation reactions in a catalytic membrane reactor", Catalysis Today, 56, 3-11 (2000). [ Links ]
38. Wieland, S., T. Melin and A. Lamm, "Membrane reactors for hydrogen production", Chemical Engineering Science, 57, 1571-1576 (2002). [ Links ]
39. Xomeritakis, G. and Y.-S. Lin, "CVD Synthesis and gas permeation properties of thin palladium/alumina membranes", AIChE Journal, 44, 174-183 (1998). [ Links ]
40. Zhang, Y., T. Ozaki, M. Komaki and C. Nishimura, "Hydrogen permeation characteristics of V-15Ni membrane with Pd/Ag overlayer by sputtering", Journal of Alloys and Compounds, 356, 553-556 (2003). [ Links ]
41. Zhang, Y., R. Maeda, M. Komaki and C. Nishimura, "Hydrogen permeation and diffusion of metallic composite membranes", Journal of Membrane Science, 269, 60-65 (2006). [ Links ]
Received: March 10, 2007.
Accepted: July 12, 2007.
Recommended by Subject Editor Orlando Alfano.














