Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Latin American applied research
versión impresa ISSN 0327-0793
Lat. Am. appl. res. v.38 n.3 Bahía Blanca jul. 2008
Enantioselective extraction of mandelic acid enantiomers using ester alcohol L-tartarate as chiral selector
F. P. Jiao1,2, X. Q. Chen1, L. Yang and Y. H. Hu2
1 School of Chemistry & Chemical Engineering,
2 School of Minerals Processing & Bioengineering, Central South University, Changsha 410083, China
jiaofp@163.com
Abstract — Distribution behavior of mandelic acid enantiomers was examined in an aqueous-organic solvent of a two-phase system containing L-tartarate. The influences of pH, length of alkyl chain of L-tartarate, organic solvent, concentration of phosphate salt and time of chiral extraction on partition coefficient (k) and separation factor (α) were investigated, respectively. With the rise of pH, k decreases, but α increases. With the addition of length of alkyl chain of L-tartarate, k and α decrease. The organic solvent and concentration of phosphate salt also have a bigger influence on k and α. A new binary chiral selector system is presented, effective for the enantioselective extraction of racemic mandelic acid. The distribution coefficients of D- and L-mandelic acid as high as 14.9 and 7.0, respectively, and the enantioselectivity value of 2.1 were found.
Keyword — Mandelic Acid Enantiomers. L-Tartarate. Chiral Extraction. Binary Chiral Selector.
I. INTRODUCTION
As the relation of chirality and drug activity is researched deeply, people become to realize the clinic importance of chirality. The different enantiomers of a drug can have vastly different pharmacological activities, pharmacokinetic processes and toxicity (Herráez-Hernández and Campíns-Falcó, 2000; Mostafavi and Foster, 2002). The most well documented example is that of the drug substance thalidomide. Bitter lessons and scientific research promote the interest in single-enantiomer drugs, so the potential of the chiral drug market is enormous (Cannarsa, 1996). How to obtain stereochemically pure drugs becomes one of the top-topics in the world. At present, chiral drugs represent above 50% of the total number of sales, but nearly 85%-90% of them are racemic mixtures because of the difficulties of separation techniques. So, preparative separations of enantiomers are very important (Seo et al., 2000).
To separate enantiomers, techniques such as crystallization, enzymatic conversion and chromatography have been developed. The techniques promote the research and development of chiral compounds, but there are some deficiencies about them. Crystallization is time- and cost-inefficient, and very often confined to racemic compounds such as acids and bases. Enzymatic conversion is expensive due to its single-action. As chiral stationary phases, mobile phases and derivatizing agents are very expensive, chromatography is cost-inefficient. To avoid the problems above, asymmetric synthesis and kinetic resolution have been developed (Rekoske, 2001; Cen and Cai, 2000; Prelog et al., 1989). However, it is very expensive and time-inefficient to develop a proper route for every chiral compound. Solvent extraction is a mature industrial separation technique that can be easily performed automatically and continuously with good effect, high recovery and low energy consumption. Chiral solvent extraction is a very attractive option for separation of enantiomers (Lacour et al., 2000; Heldin et al., 1991).
Mandelic acid (MA) served as a model in this study, which is a major metabolite of styrene and widely used as a biological indicator of occupational exposure to styrene (Horváth et al., 2000).
Diester derivatives of tartaric acid are well known as effective chiral selectors. Because these derivatives are symmetric in the C2 axis and two kinds of functional groups, hydroxyl and carbonyl, attached to asymmetric carbons in these derivatives are stereochemically equivalent to those groups attached to other carbons; these structural features are favorable for them to be chiral selectors (Heldin et al., 1991). MA has a carboxylic acid group at the chiral center that could participate in additional interactions with the hydroxyl and carbonyl groups of the enantiopure di-tartarates.
In this paper, the distribution behavior of MA enantiomers was examined in the aqueous and organic solvent of a two-phase systems containing L-tartarate. Finally, a new binary chiral selector system, effective for the enantioselective extraction of racemic mandelic, is presented.
II. METHODS
A. Chemicals
Racemic MA was supplied by Sigma (St. Louis, MO, USA). β-CD was purchased from Abxing Biological Technology Co. Ltd (Beijing, China). Other chemicals were of analytical reagent grade.
B. Analytical method
Chromatographic studies were performed using a LC-2010 HPLC (Shimadzu, Japan). The mobile phase was water-methanol mixture (Δr=85:15) containing 6 mmol/L L-phenylalanine and 3 mmol/L CuSO4 and running at a flow rate of 0.6 mL/min. The column (250 mm × 4.6 mm i.d.) was packed with 5 μm RP Cl8. The UV spectrometer was operated at 269 nm.
C. Enantioselectivity experiments
The enantioselectivity (α) is defined as the ratio (kD / kL) of both distribution coefficients of D-enantiomer to L-enantiomer in an aqueous-organic two-phase system containing chiral selector. In a 60 mL separatory funnel, 10 mL of an aqueous solution containing an aqueous phosphate buffer and 1.28 g/L of racemic MA were shaken for some time with 10 mL of the organic phase containing 0.31 mol/L chiral selector at the temperature of 22 °C. After the two phases were separated, the concentrations of D- and L-enantiomer were measured by HPLC. From the results, kD, kL and α can be obtained.
III. THEORY
D- and L-enantiomers can form two diastereomeric complexes with L- or D-chiral selector (CS) through coulombic interactions, hydrogen bonding and van der Waals interaction in a chiral system, which are described as follows:
L + L - CS ⇔ L - L - CS (1)
D + L - CS ⇔ D - L - CS (2)
or
L + D - CS ⇔ L - D - CS (3)
D + D - CS ⇔ D - D -CS. (4)
The stability of the two complexes (L-L-CS and D-L-CS or L-D-CS and D-D-CS) is different in lipophilic organic phase, which can be represented by the difference in free energies of partition -Δ(ΔG). It can be deduced by the following equation:
 | (5) |
Only if α is not equal to 1, different degrees of separation of racemate can be achieved by chiral extraction.
IV. RESULTS AND DISCUSSION
According to the above-mentioned theory, the influences of pH, organic solvent, length of alkyl chain of L-tartarate, concentration of phosphate salt and time of chiral extraction could affect the partition coefficient (k) and separation factor (α). To make the experiment data comparable, the concentration of MA were fixed at 1.28 g/L, and the influence of other factors were studied.
A. The effect of buffer pH
PH has been reported to be a parameter affecting the resolution in a chiral extraction system (Coelhoso et al., 2000). To investigate the effect of buffer pH on k and α, the distribution behavior of MA enantiomers is examined in the aqueous and organic solvent of a two-phase system containing 0.31 mol/L L-pentyltartarate, 0.1 mol/L Na2HPO4/H3PO4 buffer at different pH and n-decanol as organic solvent. The results obtained are shown in Fig.1. With the increase of buffer pH, the k decreases obviously. Because MA has a carboxylic acid functionality, the molecule dissociates in a aqueous solution releasing a proton under the conditions of the higher buffer pH. So ionization suppression controlled by pH, the lower buffer pH in favor of the chiral extraction. However, the α increases with the increase of buffer pH, that is to say, the effect of buffer pH of kD is more obvious than that of kL.
B. The effect of concentration of phosphate
The phosphate concentration is also a parameter affecting the resolution in a chiral extraction system. To study the effect of phosphate concentration on k and α, the distribution behavior of MA enantiomers is examined in the aqueous and organic solvent of a two-phase system containing 0.31 mol/L L-pentyltartarate, Na2HPO4/H3PO4 buffer (pH 2.3) and n-decanol as organic solvent. The k increases with the increase of salt concentration, the α decreases with the increase of buffer concentration, which might be related to the influence of the activity of aqueous solution, because of the variety of phosphate concentration. In general, the concentration of MA in aqueous phase will decrease because with the increases of phosphate concentration, the activity of aqueous solution decreases.

Fig.1 Influence of pH on k and α
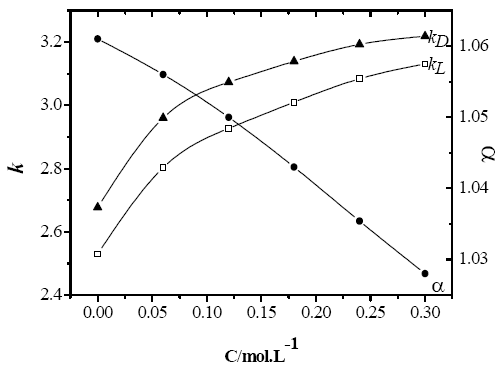
Fig.2 Influence of concentration of phosphate salt on k and α
C. The effect of organic solvent
Chiral extraction performance is not only related to the structure of chiral selector, but also to the properties of organic solvent. So it is very important to investigate the influence of different organic solvent on k and α of MA enantiomers, containing 0.1 mol/L Na2HPO4/H3PO4 buffer (pH 2.3), 0.31 mol/L L-pentyltartrate. From Table 1, we can see that the extraction efficiencies for the different organic solvents are quite different. The distribution ratios of MA enantiomers is almost ignored employing n-heptane with the stronger hydrophobic performance as the organic solvent, and that of with 1, 2-dichloroethane is between that of with n-heptane and that of with other alcoholic complexes. The results listed in Table 1 clearly reveal that the partition coefficient decreases and the enantioselectivity increases with the addition of length of alkyl chain of alcohol. It might be related with the polarity and interacts of different organic solvent with solute
D. The effect of different alkyl chain of L-tartarate and binary chiral selector
Extraction of racemic mixtures is dependent on the difference in stability of the two diastereomeric complexes which are formed by chiral selector with the two enantiomers. So chiral extraction performance is related to the structure of chiral selector (Keurentjes et al., 1996). It is very important to study the influence of different alkyl chains of L-tartarate on k and α of both MA enantiomer, with the chiral selector concentration of 0.31 mol/L, 0.1 mol/L Na2HPO4/H3PO4 buffer (pH 2.3), and n-decanol as organic solvent. It is seen from Table 2 that kD is always bigger than kL, that is to say, the complexes of L-tartarate with D-MA are more stable than that of L-tartarate with L-MA, which is related to the lower spatial resistance of L-tartarate with D-MA. The k and α decrease with the addition of length of alkyl chain of L-tartarate, which was induced by the decreases of stability of complexes of L-tartarate with MA enantiomers. Because the polarity decreases, the hydrophobic nature and steric resistance increase with the addition of different length of alkyl chain of L-tartarate. This feature also can be seen from the difference of extraction reaction free energy -Δ(ΔG) of two enantiomers.
However, the values of partition coefficient and separation factor are low for most traditional single extraction in Table 2. A possible improvement in single chiral extractions may be achieved by combining L-tartrates with other chiral selector. β-Cyclodextrin (β-CD) has been used successfully to separate enantiomers of numerous substances, both as a chiral mobile phase and chiral stationary phase selector. Particular advantages of β-CD are its chemical stability, host-guest complexation ability, relatively high water solubility, low price and availability (Jiao et al., 2007). The performance of β-CD and L-pentyltartarate as a binary selector system was studied under the extraction conditions of 0.31 mol/L L-dipentyltartrate and β-CD concentrations of 3 mmol/L, while L-pentyltartarate and β-CD had a low enantioselectivity as single chiral selector. A preferential extraction of D-MA to the organic phase was found in the binary selector system. An important increase of the values of distribution coefficients and enantioselectivity was observed in comparison with the values determined for L-pentyltartarate. The distribution coefficients of D- and L-MA as high as 14.9 and 7.0, respectively, and the enantioselectivity value of 2.1 were found. A possible explanation of this behavior is that the aromatic ring of MA could be incorporated into the β-CD cavity while the carboxylic and hydroxyl groups of the chiral center of MA interacted with the secondary hydroxyl groups of the β-CD cavity. Presumably, β-CD preferentially interacts with D-MA, thus accelerating its transfer into the organic phase where D-MA interacts with L-pentyltartarate. This would increase the driving force for the transfer of D-MA and improve the chiral resolution. Simultaneously, soluble β-CD could facilitate the solubility of MA in aqueous phase. The better binary chiral selector system also apply to the other L- or D- tartarate with β-CD at the same time.
E. The effect of time of chiral extraction
During the course of the chiral extraction, the reversible reactions that L-tartrate form diastereomeric complexes with D- and L-MA by non-covalent bonding are involved. In general, the reversible reactions are slow. Therefore, it is necessary to investigate the equilibrium time to evaluate the separation ability of chiral extraction containing 0.31 mol/L L-pentyltaratrate, 0.1 mol/L Na2HPO4/H3PO4 buffer (pH 2.3) and n-decanol as organic solvent. It is found that the chiral extraction reaches equilibrium after 1 h.
Table 1 Influence of organic solvents on k, α and - Δ(ΔG)

Table 2 Effect of length of alkyl chain of L-tartarate on k and α

V. CONCLUSIONS
Chiral solvent extraction is a very attractive option for separation of enantiomers, especially using the superior chiral selector. The distribution behavior of MA enantiomers was examined in a two-phase systems containing L-tartarate. The L-tartarate form more stable complexes with D-MA than that with L-MA, the partition coefficient and enantioselectivity of racemic MA decrease with the addition of length of alkyl chain of L-tartarate, solvent and phosphate concentration have a large influence on distribution behavior, optimum pH is about 2.3 for separation of racemic MA. The performance of β-CD/L-pentyltartarate as a new binary selector system was studied. An important increase of the values of distribution coefficients and enantioselectivity was observed in comparison with the values determined for L-pentyltartarate. The chiral extraction reaches equilibrium after 1 h.
VI. ACKNOWLEDGEMENTS
This work was supported by the Natural Science Foundation of China (20776162) and by the Postdoctoral Science Foundation of Central South University
REFERENCES
1. Cannarsa, M.J., "Single enantiomer drugs: new strategies and directions", Chemistry & Industry, 5, 374-378 (1996). [ Links ]
2. Cen, Z.Z. and S.H. Cai, "Solvent extraction of ephedrine epimerides with chiral agents", J. Chem. Ind. Eng. (China), 51, 418-420 (2000). [ Links ]
3. Coelhoso, I.M., M.M. Cardoso, R.M.C. Viegas and J.P.S.G. Crespo, "Transport mechanisms and modelling in liquid membrane contactors", Sep. Purif. Technol., 19, 183-197 (2000). [ Links ]
4. Heldin, E., K.J. Lindner, C. Pettersson, W. Lindner and R. Rao. "Tartaric acid derivatives as chiral selectors in liquid chromatography", Chromatographia, 32, 407-416 (1991). [ Links ]
5. Herráez-Hernández, R. and P. Campíns-Falcó, "Chromatographic separation of chlothalidone enantiomers using β-cyclodextrins as chiral additives", J. Chromatogr. B, 740, 169-177 (2000). [ Links ]
6. Horváth, E., J. Kristóf, R.L. Frost, L. Rintoul, Á. Rédey and W. Forsling, "Investigation of mandelic acid bonding on Pirkle type chromatographic stationary phases by Raman spectroscopy", J. Chromatogr. A, 893, 37-46 (2000). [ Links ]
7. Jiao, F.P., X.Q. Chen and W.G. Hu, "Enantioselective extraction of mandelic acid enantiomers by L-dipentyl tartrate and β-cyclodextrin as Binary chiral Selectors", Chem. Pap., 61, 326-328 (2007). [ Links ]
8. Keurentjes, J.T.F., L.J.W.M. Nabuurs and E.A. Vegter, "Liquid membrane technology for the separation of racemic mixtures", J. Membr. Sci., 113, 351-360 (1996). [ Links ]
9. Lacour, J., C. Goujon-Ginglinger and S. Torche-Haldimann, "Efficient enantioselective extraction tris(diimine)-ruthenium(ii) compexes by chiral, lipophilic TRISPHAT anions", Angew. Chem., 39, 3695-3697 (2000). [ Links ]
10. Mostafavi, S.A. and R.T. Foster, "Pharmacokinetics of metoprolol enantiomers following single and multiple administration of racemate in rat", Internal. J. pharm., 2, 97-102 (2002). [ Links ]
11. Prelog, V., M. Kovacevic and M. Egli, "Lipophilic tartaric acid esters as enantioselective ionophores", Angew. Chem., 28, 1147-1152 (1989). [ Links ]
12. Rekoske, J.E., "Chiral Separations", AIChE J., 47, 2-5 (2001). [ Links ]
13. Seo, J.S., D. Whang, H. Lee, S.I. Jun, J. Oh, Y.J. Jeon and K. Kim, "A homochiral metal-organic porous material for enantioselective separation and catalysis", Nature, 404, 982-986 (2000). [ Links ]
Received: October 19, 2006.
Accepted: November 3, 2007.
Recommended by Subject Editor José Pinto.














