Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Latin American applied research
Print version ISSN 0327-0793
Lat. Am. appl. res. vol.39 no.1 Bahía Blanca Jan. 2009
ARTICLES
Conversion of rice husk ash into zeolitic materials
E.L. Foletto*, M.M. Castoldi, L.H. Oliveira, R. Hoffmann and S.L. Jahn
Chemical Engineering Department, Federal Universtiy of Santa Maria, 97150-900, Santa Maria,RS, Brazil
* foletto@smail.ufsm.br
Abstract - The objective of this work was to synthesize zeolitic products using rice husk ash (RHA) as silicon source. For the synthesis, two mineralizing agents: NaOH and KOH were tested. Chemical composition of the synthesized samples as well as their cation exchange capacity (CEC) were determined. The samples were also characterized by X-ray diffraction (XRD) and scanning electron microscopy (SEM). Formation of structure LTA (zeolite A) with NaOH as mineralizing agent was verified. The zeolitic materials presented high capacity of cation exchange, in the range of 5-6 meq/g.
Keywords -Rice Husk Ash. Zeolite. Synthesis
I. INTRODUCTION
Due to high cation exchange capacity (CEC), as well as small size particles, type A zeolite is widely used in water treatment, in particular for incorporation into the composition of detergents for the sequestration of calcium from wash water (Strack, 1981, Allen et al., 1983). However, to make available this application, the cost of the zeolite must be low, demanding the utilization of cheap raw materials for the production process. In the state of Rio Grande do Sul (Brazil), the rice production is about 5,137 million t per year. Since the husks represent 20% of this value, the annual production of this residue in the state is approximately 1.027.400 t. These husks are burnt for energy production, generating great amounts of ashes (~200,000 t per year). As the RHA presents > 90% by weight in silicon, its use as silicon source in the synthesis of zeolite A constitutes an alternative for reduction of costs. The RHA can be used as adsorbent in the process of gold extraction, in the production of silicon carbides (SiC), as load in polymers, as additive for cement, in the manufacture of concrete, and as support for preparation of nickel-based catalysts (Foletto et al., 2005). The ashes have been also used for zeolite synthesis: ZSM-5 (Rawtani et al., 1989, Kumar and Das, 1992, Chareonpanich et al., 2004), mordenite (Bajpai et al., 1981), ZSM-48 (Wang et al., 1998), NaX (Hamilton et al., 1993, Dalai et al., 1985), beta (Prasetyoko et al., 2005), and omega (Fajula et al., 1987).
This work had as objective to synthesize zeolitic materials using rice husk ash as silica source. Two different alkalis were used and their influence on the zeolite formation was examined.
II. MATERIALS AND METHODS
A. Composition of the Reaction Mixture
For the formation of the zeolite in alkaline pH, the molar composition proposal by Thompson and Huber (1982) was taken as reference: 1Al2O3:2.1SiO2: 3.9M2O.128H2O, where M represents sodium or potassium. The Si/Al ratio used in the reaction medium was 1.05. As silicon source, rice husk ash generated from an industrial burner of a local industry (INDUBER, Santa Maria, RS, Brazil) was used. As alkali source, NaOH and KOH were used. As aluminum source, sodium or potassium aluminates were used. They were prepared by dissolution of 54 g aluminum wire (1mm diam., 99.99%, Aldrich) in 2615 mL of sodium hydroxide solution (12% by weight) or in 2584 mL of potassium hydroxide solution (11% by weight), using a heating system with reflux. The time of reflux was 30 minutes.
B. Crystallization
The preparation of the reaction mixture was carried out in accordance with the following procedure: 2.07 g of ashes were conditioned in stainless steel autoclaves, provided internally with Teflon vessels of 40 cm3. 40 g of sodium or potassium aluminate solution was added on this ash; the autoclaves were closed and introduced into a pre-heated oven at the temperature of crystallization (100 °C). The crystallization was performed for different periods: 3, 6 and 12 h. The formed solid was separated from the supernatant liquid by filtering and was washed with deionized water to remove the excess of alkali. Later, it was dried at 110 °C for 10 h. The samples are designated as N3, N6, N12, K3, K6, K12 (N and K represent the used mineralizing agents, NaOH and KOH, whereas e 3, 6 and 12, indicate the period used for the reaction process).
C. Samples Characterization
The RHA and the samples of aluminosilicates synthesized were characterized by X-ray diffraction (Shimadzu diffractometer, model XD-7A, with radiation Cu-Kα) and by scanning electron micrograph (SEM; Model 2000FX, JEOL Co.). The chemical composition was determined by atomic absorption spectrometry (Analytik JENA, Vario 6, Germany). For determination of the cation exchange capacity, 0.5g sample was placed in contact with NH4+ excess (by the use of ammonium acetate), washed with ethanol and later calcinated for release of ammonia. The set free ammonia was collected in water and this solution was then titrated with H2SO4, expressing the values in meq/g of sample.
III. RESULTS AND DISCUSSION
The chemical composition of the RHA used in the assays was (% by weight): 94.4 (SiO2), 1.21 (MgO), 1.06 (K2O), 0.83 (CaO), 0.77 (Na2O), 0.61 (Al2O3), 0.59 (MnO), 0.03 (Fe2O). It can be observed that the from the burning of rice husks has high SiO2 content and small amounts of other substances considered as impurities.
The RHA diffractogram (Fig. 1) indicates that the same one is formed by silica in the crystalline form, resulting from the predominant presence of cristobalite (2q = 21.9) (Shinohara and Kohyama, 2004). The crystalline, amorphous or both silica forms depend on the burning temperature or the method used for ash production. When the burning temperature of RHA is high, the silica contained in the ash is predominantly crystalline.

Fig. 1. X-ray diffraction of RHA (C = cristobalite; Q = quartz).
In Table 1, the results of chemical analysis for the different samples using NaOH and KOH as mineralizing agents are shown.
Table 1. Chemical analysis of samples.

* Molar ratio.
It was evidenced that the samples synthesized in the presence of NaOH showed a small increase in Al2O3 content with the crystallization time, making the Si/Al ratio to decrease. However, the values of the Si/Al ratio in the solid are superior to that used in the reaction medium (Si/Al = 1.05). This result shows that the aluminum added to the reaction mixture is not completely incorporated in the solid at the conditions used in these assays, probably needing longer times. The samples synthesized with KOH presented a Al2O3 content slightly smaller than that of the samples synthesized with NaOH. The amount of this oxide tends to increase with the crystallization time making the Si/Al ratio to diminish. Even presenting a lower Al2O3 content, the samples synthesized with KOH showed minor Si/Al ratio in relation to the ones synthesized with NaOH. Such behavior may be due to the highly alkaline property of KOH which can dissolve faster the alumina and the silica from the RHA.
In Figures 2 and 3, the X-ray diffractogram for the samples crystallized for 3 and 12h are presented. Comparison of the diffractogram of Fig. 2 with literature patterns (Rakoczy and Traa, 2003) indicates the formation of zeolite A. The N12 sample was more crystalline in relation to N3 sample, indicating that the reaction time increases the crystallinity of the formed solids. The samples synthesized with KOH as mineralizing agent had been presented the amorphous form (Fig. 3), as indicated by the rise in the band of diffraction (in the range 2q = 20-40°). It can be observed that when potassium is used as compensation and structure-direct agent, the formation of a well defined crystalline structure does not occur. These results show that the presence of K+ cation in the reaction mixture makes difficult the formation of the crystalline structure. This fact is not verified when Na+ cation is used. The formation of an amorphous form is due to the fact that potassium presents a capacity to complex water around it lower than sodium ions, making difficult the formation of the necessary secondary units for the conception of a well defined crystalline structure.
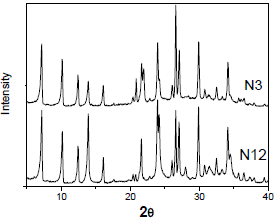
Fig. 2. X-ray diffractograms of the samples N3 and N12.

Fig. 3. X-ray diffractograms of the samples K3 and K12 (C = crstobalite; Q = quartz).
In Figs. 4 and 5 the SEM micrographs of the samples synthesized with NaOH and KOH are presented, respectively. As observed Figure 4 the particles are formed by accumulations of crystallites. For the N12 sample (Fig 4.b), the crystals present more rounded and bigger shape compared with the ones of the N3 sample (Fig 4.a). The results indicated that the prolongation of the crystallization time leads to bigger crystals for NaOH. The samples synthesized with KOH (Fig. 5) also presented particle accumulations; however, the size of the particles tends to diminish with the increase of the crystallization time, indicating a re-crystallization in the system. Accordingly, the type of cation involved in the mineralizing agent influences directly on nucleation of the system.


Fig. 4. SEM micrographs of the samples: a) N3 and b) N12. (Magnification: 5000x)

Fig. 5. SEM micrographs of the samples: a) K3 and b) K12. (Magnification: 5000x)
In Table 2, results of cation exchange capacity (CEC) for the samples synthesized with NaOH and KOH are listed.
Table 2. Results of CEC for samples synthesized with NaOH and KOH.

It is verified that all the samples presented a well superior CEC to the one of the RHA (0.12 meq/g). The CEC decreased with the increase of crystallization time for the samples prepared with NaOH as well as KOH. Compared to CEC results reported for other aluminosilicates, 3-4 meq/g (Bretaudeau et al., 1995; Deabriges, 1982; Krummel and Gault, 1976), it may be observed that the values obtained in this work are rather superior.
IV. CONCLUSIONS
It was feasible to synthesize zeolite A using RHA as silicon source and NaOH as mineralizing agent. The synthesis carried out with KOH led to the formation of amorphous aluminosilicates, with high cation exchange capacity. All the samples probably present good adsorptive properties accounting for the high values of CEC. Accordingly, they are potentially suited to be used as exchanger of cations in the formularization of detergents and for effluents treatment. Due to high potassium content, the solids yielded from the KOH reaction present potential to be used as source of this cation for ground corrective fertilizer formularizations with the advantage on the conventional methods to slowly liberate the potassium in the ground, preventing leaching.
ACKNOWLEDGEMENTS
The authors are grateful to the Brazilian research funding, CNPq and FAPERGS, for the financial support.
REFERENCES
1. Allen, H.E., S.H. Cho and T.A. Neubecker, "Ion exchange and hydrolysis of type A zeolite in natural waters," Water Resources, 17, 1871-1879 (1983). [ Links ]
2. Bajpai, P.K., M.S. Rao and K.V.G.K. Gokhale, "Synthesis of mordenite type zeolite using silica from rice husk ash," Industrial & Engineering Chemistry Product Research and Development, 20, 721-726 (1981). [ Links ]
3. Bretaudeau, D., F. Delprato and M. Malassis, "Preparation of crystalline 4A zeolites," U.S. Patent 5,474,753 (1995). [ Links ]
4. Chareonpanich, M., T. Namto, P. Kongkachuichay and J. Limtrakul, "Synthesis of ZSM-5 zeolite from lignite fly ash and rice husk ash," Fuel Processing Technology, 85, 1623-1634 (2004). [ Links ]
5. Dalai, A.K., M.S. Rao and K.V.G.K. Gokhale, "Synthesis of NaX zeolite using silica from rice husk ash," Industrial & Engineering Chemistry Product Research and Development, 24, 465-468 (1985). [ Links ]
6. Deabriges, J., "Industrial process for continuous production of zeolite A," U. S. Patent 4,314,979 (1982). [ Links ]
7. Fajula, F., M.V.-Pacheco and F. Figueras, "Synthesis of zeolite omega - Influence of the temperature and the reagents on the crystallization kinetics," Zeolites, 7, 203-208 (1987). [ Links ]
8. Foletto, E.L., R. Hoffmann, R.S. Hoffmann, U.L. Portugal Jr. and S.L. Jahn, "Aplicabilidade das cinzas da casca de arroz," Química Nova 28, 1055-1060 (2005). [ Links ]
9. Hamilton, K.E., E.N. Coker, A. Sacco Jr., A.G. Dixon and R.W. Thompson, "The effects of the silica source on the crystallization of zeolite NaX," Zeolites, 13, 645-653 (1993). [ Links ]
10. Krummel, H. K. and T.W. Gault, "Detergent compositions," U. S. Patent 3,985,669 (1976). [ Links ]
11. Kumar, N.L. and D. Das, "Zeolite (ZSM-5) synthesis from rice husk for xylene isomerization," Research and Industry, 37, 3, 141-142 (1992). [ Links ]
12. Prasetyoko, D., Z. Ramli, S. Endud, H. Hamdan and B. Sulikowski, "Conversion of rice husk ash to zeolite beta," Waste Management, 26, 1173-1179 (2005). [ Links ]
13. Rakoczy, R.A. and Y. Traa, "Nanocrystalline zeolite A: synthesis, ion exchange and dealumination," Microporous and Mesoporous Materials, 60, 69-78 (2003). [ Links ]
14. Rawtani, A.V., M.S. Rao and K. Gokhale, "Synthesis of ZSM-5 using silica from rice husk ash," Industrial & Engineering Chemistry Research, 9, 1411-1414 (1989). [ Links ]
15. Shinohara, Y. and N. Kohyama, "Quantitative analysis of tridymite and cristobalite crystallized in rice husk ash by heating," Industrial Health, 42, 277-285 (2004). [ Links ]
16. Strack, H., "Crystalline zeolite powder of type A," U. S. Patent 4,303,629 (1981). [ Links ]
17. Thompson, R.W. and M.J. Huber, "Analysis of the growth of molecular sieve zeolite NaA in a batch precipitation system," Journal of Crystal Growth 56, 711-722 (1982). [ Links ]
18. Wang, H.P., K.S. Lin, Y.J. Huang, M.C. Li and L.K. Tsaur, "Synthesis of zeolite ZSM-48 from rice husk ash," Journal of Hazardous Materials 58, 147-152 (1998). [ Links ]
Received: August 2, 2006.
Accepted: May 16, 2008.
Recommended by Subject Editor Ana Lea Cukierman.














