Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Latin American applied research
versión impresa ISSN 0327-0793
Lat. Am. appl. res. vol.39 no.4 Bahía Blanca oct. 2009
ARTICLES
Evaluation of the adsorptive quality for Ni and v of kraft lignin under different conditions
R. Urbina, J. Casanova, N. Pérez and J. Fernández
Grupo TECALL, Laboratorio de Carbón y Residuales de Petróleo.
Dpto. de Procesos y Sistemas, Univ. Simón Bolívar, 1080 Caracas, Venezuela
naperez@usb.ve
Abstract - In this study, the impact of the precipitation process over the adsorption capability of Nickel and Vanadium of precipitated lignins of Kraft black liquor was studied. The studied conditions of precipitation were: mixing (shaking and stirring), mixing speed and mixing sequence. To determine the best lay out for the studied conditions, the quantity of produced adsorbent by precipitation and its adsorption capability were correlated. It was determined that the type of mixing in the process of precipitation affects the adsorption capability of lignins and conditions for a higher adsorption capability of each of the studied metals were determined.
Keywords - Adsorption; Mechanical Conditions; Lignin; Heavy Metals.
I. INTRODUCTION
The industrial dumping of heavy metals directly into lakes and rivers constitutes a serious environmental problem which highly affects the quality of water. When concentration of these metals in the water exceeds certain values, they become a health risk. Also, environmental legislation in many countries demands low levels of these metals. As a consequence, industries are forced to look for efficient and economic solutions to achieve the goal of reducing these levels (Basso et al., 2002).
For paper manufacturing, it is necessary to separate cellulose fibers, strongly joined by lignin (a resinous adhesive which provides structural support to the tree), for then being able to produce the pulp, which can be obtained either by chemical or mechanical methods.
Mechanical methods are based on pressing trunks in a mill of radial bar discs which turn at high speed with the presence of abundant water. These processes have always represented an important part in the world production of cellulose pulp, for their overall lower cost, better use of resources, and lower level of pollution. (Area, 2005).
In chemical methods, the wood is cooked in a solution of chemical compounds. The resultant effluents of the baking of wood (black liquor) have many pollutant agents, and they are often treated, purified and recycled to recover sodium sulfide and caustic soda.
The research of new technologies that involve removal of poisonous metals from residual waters focuses its attention in biosorption, a technology based on the aptitude to connect metals to diverse biological materials, e.g. lignin, seaweeds, bacterial biomass, etc., and which has been used as a way to recover heavy metals. These biopolymers contain a variety of functional groups capable to adsorb ionic species of a size and specific load.
In order to find alternative resources, and to maximize the use of the ones available, the group Tecnologías Alternativas Limpias TECALL (Alternative Clean Technologies) of Universidad Simón Bolívar has conducted diverse research, where they have used lignins in order to remove such heavy metals as Nickel and Vanadium in aqueous solutions coming from acid digestion of coke via microwave (Pérez et al., 2007).
González (2005) allowed verifying that lignins possess high affinity towards these metals. In this study, the Kraft black liquor was used as raw material for obtaining lignins by precipitation mechanism, obtaining good results.
One of the ways for reducing emissions in the aquatic means is the use of Kraft black liquor as raw material by industries. Recent studies by Núñez (2006) and Hernández (2007), have rushed lignin from Kraft black liquor, in order to obtain a low cost adsorbent that allows the removal of metals such as Nickel and Vanadium from aqueous solutions, also leading to determining appropriate conditions like pH, type of precipitation agent, and concentration of the same one for lignin precipitation from the Venezuelan Kraft black liquor.
This study focuses on improving the quality of lignin as an adsorbent for Nickel and Vanadium changing precipitation conditions, in order to obtain a low-cost adsorbent which allows the removal of these metals, in this way reducing environmental detriment, and giving value to black liquor which currently is a paper industry waste.
The effect of kind of mixing was studied; specifically shaking and stirring, as well as mixing speed, reactive mixture order, acid consumption in the process, and finally, the adsorbent selectivity for solutions of ionic Nickel and Vanadium.
In order to define the most effective method, the parameter lignin quality was used, which allows determining the best relation between produced mass and adsorptive capability, to find the best conditions to use for preparation of the adsorbent.
The term lignin quality as defined by Hernández (2007) relates quantity of produced adsorbent in the precipitation with the adsorptive capability of the same. "Lignin quality for X metal" was defined (from now on referred to as "quality"), as it appears on Eq. 1:
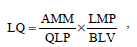 | (1) |
where LQ is Lignin quality expressed in milligrams of the adsorbed metal divided by milliliters of Kraft black liquor, AMM (mg) is the mass of metal removed by the adsorbent, LMP (mg) is the lignin mass obtained by precipitation, QLP (mg) is the lignin quantity used in the adsorption process, BLV (ml) is the Kraft black liquor volume used to obtain lignin under stated conditions.
II. METHODS
This research was based on the study of the precipitation process and its effect on adsorption, in order to determine the mechanical conditions that allow to obtain better quality lignins.
Study variables which affected the precipitation conditions were:
1. Mixing: two types of mixing were used, shaking or swaying on axis, and stirring or circular mixing counterclockwise.
2. Mixing speed: Studies were performed at speeds of 100 rpm and 800 rpm.
3. Mixture order: Two mixture sequences were evaluated: adding the precipitation reagent (acid) to the black liquor and vice versa.
First, the Kraft black liquor was in contact with the precipitation reactant under conditions shown in Table 1. These conditions were determined by Hernández (2007). That research studied the conditions for lignin precipitation to achieve the best adsorption of Nickel and Vanadium. The precipitate was filtered through gravity using filter paper, washed with distilled water and then dried off with a lamp for an hour and a half under a temperature below 80° C, to avoid charring. The lignin fraction thus obtained was crushed and weighed.
Table 1. Conditions for lignin precipitation to reach the best adsorption of Nickel and Vanadium. (Hernández, 2007)

Lignin fractions obtained from precipitation were subject to an adsorption process under conditions presented in Table 2, from 20 ppm solutions for Nickel and Vanadium in order to determine their adsorptive capability. The adsorption process was carried out with a batch system. First, the solution was taken to the required pH by acid addition, and then, the adsorbent mass was added. The process was carried out under constant mixing for 2 h. Both the initial and final concentrations of metals were measured by Inductively Coupled Plasma Mass Spectrometry (ICPMS).
Table 2. Adsorption conditions for Nickel and Vanadium for lignin. (Pérez et al., 2006)

III. RESULTS AND DISCUSSIONS
A. Effect of the mixing and mixing speed on the absorptive capability in lignin precipitation.
Nickel
Table 3 shows summed up experiments on the effect of speed and the kind of mixing in adsorptive capability and quality.
Table 3. Effect of speed and type of mixing in Nickel adsorption

According to results, measurable differences exist in the adsorptive capability of lignins by changing both the kind of mixing, and the mixing speed. These differences affect lignin quality which depends on mechanical conditions used for precipitation. These can generate different grains for each lignin, with more or less affinity to Nickel.
Similar results were reported by Pasquini et al. (2002) who proposed that lignin molecules tend to organize themselves on substrate, and form aggregates depending on the type of substratum and its interactions, considerably affecting the presence of polar groups in the surface. This author considered that controlling variables such as concentration of the precipitation reactant, operation temperature, type of mixing and type of acid used, they could generate different grains for each lignin and therefore different adsorption capabilities.
For shaking, quality increases with speed, because this type of mixing, e.g. at 100 rpm allows flocculation. Nevertheless, these flocculants are not sufficiently stable as to retain an important Nickel quantity. On the contrary, at 800 rpm, they might favor the formation of grains with higher retention capability for the metal, allowing a higher Nickel adsorption; whereas lignin quality decreases with speed when stirred, because this stronger movement disperses the system, preventing the formation of floccules, and consequently, diminishing the adsorptive capability of these lignins. It is necessary to point out that on having worked with this kind of mixing at 100 rpm, and being stronger than shaking, it might improve flocculation, obtaining an increase in lignin quality.
Wang et al. (2006) studied the formation conditions, nucleation and crystal growth in the salicylic acid precipitation from polyethylenglycol solutions, thinking that the final morphology of the glazing changes with the speed of mixing. The increase in mixing speed increases the collision speed of particles, increasing agglomeration; nevertheless, at very high speeds the chipboard disintegrates. Therefore, one finds a maximum agglomeration at an intermediate speed. Changes in crystal morphology due to mixing speed can be assigned to an agglomeration phenomenon.
Vanadium
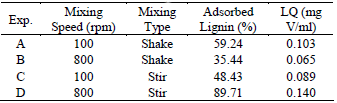
Same experiments were performed with this adsorbent to find higher affinity with Vanadium using H2SO4 0.1 M as precipitation reactant. These results are summed up in Table 4:
Table 4. Effect of speed and kind of mixing in Vanadium adsorption.
To the ends of finding conditions that would allow the formation of this kind of grain, each effect was studied to reach the right combination for the manufacturing of an adsorbent with higher affinity to Vanadium.
Regarding quality, Table 4 shows an opposite effect for Vanadium removal in shaking when comparing it with Nickel. Quality is higher than at the lowest mixing speed. Lower values for quality are directly influenced by the capability of the material to adsorb Vanadium. This confirms what exposed by Pérez et al. (2006) about adsorption mechanisms for Nickel and Vanadium.
Stir mixing shows that quality increases with mixing speed finding the maximum of 800 rpm where mechanical conditions are the highest.
Comparing both stirring and shaking for the same speed, it is found at 100 rpm that quality decreases changing from shaking to stirring while at 800 rpm quality increases with this change on kind of mixing. The highest values were found at 100 rpm for shaking and 800 rpm for stirring.
When sulfuric acid concentration decreases it may suggest an increase in polar functional groups over grain surface (Hernández, 2007). This phenomenon can be attributed to hydrogen bridges formed with solvent through the polar groups on lignin in the precipitation process, making the particles to floccule and regroup to the outer lignin surface. This fact was reported by Jirgerson (1958) where organic colloid flocculation of the aromatic rings on lignin, bencylic kind prefers to be joined as in a "sandwich", this way the grain takes a "stack" form leaving functional groups in the nearest part close to the solvent (water).
For Vanadium, the adsorbed species is metavanadate (VO3-) which has different characteristics compared with Nickel. Therefore, the adsorption mechanism for Vanadium on lignin must be totally different (Hernández, 2007). This is corroborated by the adsorption isotherms obtained by Pérez et al. (2007) which shows that the adsorption of Nickel and Vanadium presents two different mechanisms.
B. Evaluation of the effect of the reactant sequence of addition during the precipitation on adsorptive lignin capacity.
Nickel
In order to establish the best precipitation conditions of the adsorbent, a comparison was performed with all the possible conditions of production. In this case, the parameter quality was taken as decisive, due to the fact that this one provides the best adsorptive capability/produced mass. These values are shown in the Table 5. Sequence 1 corresponds to the addition of precipitation reactant (acid) to the black liquor, and the sequence 2 corresponds to the reverse case. In Table 5, it was found that precipitation reactant keeps for the two sequences; it does not imply a raise on acid volume. However, there was a difference on produced mass between sequence 1 and 2, being 1 the most productive choice.
Table 5. Effects of the change of the conditions and sequences of addition of reactant on having precipitated lignin with Nickel affinity.
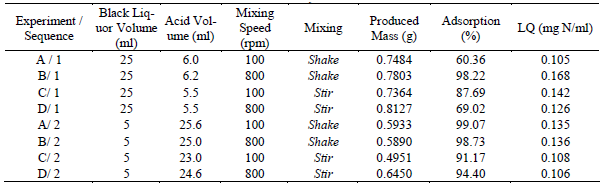
To make a decision about what sequence to use, first one has to check probable problems on mass transference. Because not all the black liquor molecules have the same probability to find the acid and its effect on the produced mass.
According to adsorptive capability, there is no big difference between each sequence, it would not be a fact in count.
In sequence 1 (acid addition), the pH solution slightly changes, rising the probability for a higher lignin quantity to fall down. However, for sequence 2 (black liquor addition), the pH change is as dramatic as, on the surface, where there formed a thin layer that closes access to higher black liquor, obtaining a minor precipitate lignin production. According to results the only effect is about the decreasing of produced mass and adsorptive capacity has no significant effect on the metal adsorption. Figure 1 shows the effect on quality.

Fig 1. Effect of study variables on quality for Nickel.
Figure 1 shows the same effect for both sequences. An increase in lignin quality for experiments A and B with shaking (Table 5), achieving a maximum value at 800 rpm, and then a decrease on experiments C and D for stirring at high speed. This effect is more remarkable for sequence 1 than for sequence 2. The highest lignin quality was reported for sequence 1.
For both sequences 1 and 2, a maximum quality of 0.168 mg Ni/ml for sequence 1, and 0.136 mg Ni/ml for sequence 2 was achieved. The mechanical conditions for these sequences were 800 rpm of mixing speed and shaking. These ones are the best mechanical conditions for Nickel adsorption.
Vanadium
To find the highest affinity for metavanadate VO3-, the same conditions were evaluated. Table 6 shows that just like in Nickel conditions, there is no meaning differences about the reactants-acid proportions, which keeps constant. But lignin quality has significant differences according to each condition, indicating a probable distinctive adsorption mechanism for Vanadium.
Table 6. Effects of the change of the conditions and sequences of addition of reactant on having precipitated lignin with Vanadium affinity.

Sequence 1 corresponds to the addition of precipitation reactant (acid) to the black liquor, and sequence 2 corresponds to the reverse case. The specific conditions reported in Table 6 for the highest Vanadium adsorption are a low mixing speed of 100 rpm (shaking) and sequence 1, and stirring at 800 rpm for sequence 2. That means that varying the reactant sequence, it modifies the lignin grain formation.
It is important to realize that acid volume for precipitation is higher because of the lower concentration of sulphuric acid (H2SO4 0.1 M). It is reasonable to think that for less acid concentration the most volume should be used.
Studying the effect of the change on reactants sequence in the adsorption of VO3- (Table 6), measurable differences appear as for the adsorptive capability of produced lignins, especially for sequence 2, where there are values as low as 9.02 %. This leads to think, that the process of adsorption of the VO3-, is so complex that any change in the conditions causes significant changes.
The precipitation of the adsorbent with affinity for Ni2+ is simpler than VO3- adsorbent, since for pH around 6, it begins to appear the precipitate, which without the suitable mixing would prevent the acid flow towards the rest of the volume of black liquor, causing that it does not go over to the wished pH of precipitation.
To establish the best conditions for securing major affinity for VO3-, the same study as in case of Ni 2+ was performed, where all possible conditions to obtain the adsorbent were combined.
As for Nickel, a graphic representation of all the conditions studied according to the quality obtained was performed on Fig. 2.
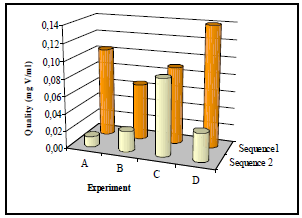
Fig 2. Effect of study variables on quality for Vanadium.
According to Fig. 2, tendencies between both sequences are completely different. For sequence 1, the highest values of quality are obtained.
In the case of Ni2+ adsorbent, for sequence 2 the produced mass is smaller than in sequence 1. However, the changes in the obtained quality are due to the change on the lignin adsorptive capability, which presents values 50 % lower than in sequence 2.
After observing the effects of sequence 1 in quality, one finds that after speed increases in shaking (experiments A and B) quality diminishes, whereas, for stirring (experiments C and D) quality increases with speed, where the adsorption reaches a value of 89.71 %.
In this setting, one keeps on supporting that conditions adapted for the securing of Vanadium adsorbent corresponds at the lowest speed shaking and the highest speed stirring, which indicates that these are the conditions that favor lignin precipitation to obtain a better quality index for VO3-.
Sequence 2 presents a different trend from that of setting 1, for this condition, it is observed that for shaking quality increases when speed increases. In this case, the quality maximum finds for stirring at 100 rpm (quality = 0.088 mg V/ml). However, sequence 2 was discarded because the obtained quality was very low in comparison to sequence 1.
Based on the previous results, stirring was selected, at a speed of 800 rpm and setting 1, to obtain an adsorbent with major affinity for Vanadium.
IV. CONCLUSIONS
- The way of mixing during precipitation does affect lignin adsorptive capacity.
- For Nickel, with the two used sequences, one found that after increasing mixing speed for shaking, obtained lignins yield better quality, while in stirring; the increase in speed produces a decrease in quality.
- Lignin quality turns out to be affected by the reactants sequence, at least for Vanadium. For sequence 1, quality diminishes with mixing speed (shaking), whereas for stirring, quality increases with this parameter, the opposite effect being observed for the setting 2.
- The volume of the precipitation reactant is maintained for the two sequences of addition of the reactants used, hence using any one of them, would not generate a higher acid consumption, however, the quality of the obtained material is affected.
- Conditions to obtain an adsorbent with the highest affinity towards Nickel are the following ones: sequence 1, mixing speed of 800 rpm and a type of agitation shake.
- Conditions to obtain an adsorbent with the highest affinity towards Vanadium are the following ones: sequence 1, mixing speed of 800 rpm and stirring.
ACKNOWLEDGMENTS
The authors would like to acknowledge financial support from Universidad Simón Bolívar; the National Fund of Science Technology and Innovation of Venezuela (FONACIT) by means of the project N° 2005000432; and the Alfa Program Lignocarb Alfa II 0412FAFI. The authors are thankful to the CESMA-USB for the experimental design to carryout this work, to the UGA-USB for the ICP analysis.
REFERENCES
1. Area, M., "Tecnologías limpias para la producción de pulpa y papel de Eucalyptus," XX Jornadas Forestales de Entre Ríos, Concordia (2005). [ Links ]
2. Basso, M., E. Cerrella and A. Cukierman, "Lignocellulosic Material as Potentials Biosorbents of Trace Toxics Metals from Wastewater," Industrial Engineering Chemistry, 41, 3580-3585 (2002). [ Links ]
3. González, N., Estudio de adsorción de Níquel y Vanadio por ligninas, Proyecto de grado, Universidad Simón Bolívar, Sartenejas (2005). [ Links ]
4. Hernández, A., Precipitación de lignina a partir de licores negros nacionales, Proyecto de grado, Universidad Simón Bolívar, Sartenejas (2007). [ Links ]
5. Jirgenson, B., Organic Colloids. Elsevier Publishing Company (1958). [ Links ]
6. Núñez, A., Preparación de un posible adsorbente de Níquel y Vanadio a partir de lignina, Trabajo de Grado, Universidad Simón Bolívar, Sartenejas (2006). [ Links ]
7. Pasquini, D., D.T. Balogh, P.A. Antunes, C.J.L. Constantino, A.A.S. Curvelo, R.F. Aroca and O.N. Oliveira, "Surface Morphology and Molecular Organization of Lignins in Langmuir-Blodgett Films," Langmuir, 18, 6593-6596 (2002). [ Links ]
8. Pérez, N., G. Rincón, L. Delgado and N. González, "Use of biopolymers for the removal of heavy metals produced by the oil industry-A feasibility study," Adsorption, 12, 279-286 (2006). [ Links ]
9. Pérez, N., M. Sánchez, G. Rincón and L. Delgado, "Study of the behavior of metal adsorption in acid solutions on lignin using a comparison of different adsorption isotherms," Latin American Applied Research, 37, 157-162 (2007). [ Links ]
10. Wang, X., J. Gillian and D. Kirwan, "Quasi-Emulsion Precipitation of Pharmaceuticals. Conditions for Formation and Crystal Nucleation and Growth Behavior," Cristal Growth & Design., 6, 2214-2224 (2006). [ Links ]
Received: February 11, 2008
Accepted: November 10, 2008
Recommended by Subject Editor: Ana Cukierman














