Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Latin American applied research
versión impresa ISSN 0327-0793
Lat. Am. appl. res. vol.40 no.1 Bahía Blanca ene. 2010
ARTICLES
Cu-doped polymeric-modified electrode for determination of cysteine
C. A. Martínez-Huitle1,2, C. Carlesi Jara3, M. Cerro-Lopez4 and M. A. Quiroz4
1 DiSTAM, University of Milan, via Celoria 2,
CAP-20133 Milan, Italy.
2 Universidade Federal do Rio Grande do Norte, Centro de Ciências Exatas e da Terra. Departamento de Química, Lagoa Nova
CEP 59078-970 - Natal, RN, Brasil. mhuitle@hotmail.com, carlos.martinez@unimi.it
3 Escuela de Ingeniería Química, Pontificia Universidad Católica de Valparaíso. Avda. Brasil 2147,
2362804 Valparaíso, Chile. carlos.carlesi@ucv.cl
4 Universidad de las Américas - Puebla. Departamento de Química y Biología, Laboratorio de Electroquímica,
CP 72820 - Puebla, México. marcoa.quiroz@udlap.mx
Abstract - A simple differential pulse voltammetric method based on chitosan modified glassy carbon was used to pre-concentrate copper ions in the chitosan film. After, this electrode was employed for the quantitative determination of cysteine. The modified electrode exhibits a clear improvement of the current response. The method allowed quantifying the analyte in the range in buffer solution and a limit of detection range from 1.0×10-6 to 1.4×10-6 M was achieved. This value seems quite higher than those previously published by other authors. The results are described and discussed in the light of the existing literature.
Keywords - Cysteine; Sensor; Chitosan; Copper.
I. INTRODUCTION
Cysteine is a naturally occurring amino acid of great importance in biological and biomedical fields. It participates in a number of biochemical processes, and is involved in several important cellular functions, such as protein synthesis, detoxification and metabolism. Cysteine is also important in food science, being widely used as antioxidant in a large variety of foods (Friedman, 1994; Elias et al., 2005; Koh et al., 1996).
Several methods have been described for quantifying cysteine in a variety of real matrices, including spectrophotometric (Zaia et al., 1999; Rozhnova et al., 1999), fluorimetric (Wang et al., 2001; Zhu et al., 2003), electrophoretic (Zeng et al., 2006; Jin and Wang, 1997) mass spectroscopy (Raftery et al., 1992) and chromatograpic (Amarnath et al., 2003; Brückner et al., 1995) ones.
Electrochemical methods were also described since offering high sensitivity and selectivity (Jin and Wang, 1997; Forsman, 1981 and 1983; Van den Berg et al., 1988; Sugawara et al., 1996; Heyrowský et al., 1997; Kawasaky et al., 1999; Amini et al., 2003). These papers deal with the behavior of cysteine at mercury electrodes (Heyrowský et al., 1997), at gold-mercury amalgam electrodes (Jin and Wang, 1997), at electrodes in the presence of Co(II) (Sugawara et al., 1996; Amini et al., 2003), at thin mercury film electrodes in the presence of osmium (Kamiñski and Modrzejewska, 1997), gold nanoparticle-modified electrodes (Agüí et al., 2007), amperometric detection (Tseng et al., 2006) and at mercury electrodes in the presence of Cu(II) (Forsman, 1981 and 1983; Van den Berg et al., 1988). According to these last papers, the reactions involved, in particular, the reaction of cysteine with Cu(II) to give a cuprous cysteinate complex and cystine can be exploited for quantifying cysteine by differential pulse cathodic stripping voltammetry. In this respect, Forsman (1981) reported that the calibration curve obtained in the presence of 10-5 M Cu(II) was linear in the 2×10-9 - 5×10-7 M range.
In the course of some investigations on the electrochemical properties of chitosan-modified (Ct-GC) electrodes, it was observed that they exhibit a high permselectivity towards Cu(II) ions (Kamiñski and Modrzejewska, 1997; Schmuhl et al., 2001; Martínez-Huitle et al., 2006). This strong affinity between Cu and chitosan film was ascribed to the presence of two ligands around each metal ion (Kamiñski and Modrzejewska, 1997; Schmuhl et al., 2001), as described in Fig. 1. Then, it seemed interesting exploring the possibility of quantifying cysteine at chitosan-Cu(II) modified glassy carbon (CtCu-GC) electrodes. This paper reports the results of some preliminary measurements.
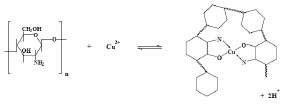
Fig. 1: Chemical structure of coordination between metal ion (Cu(II)) and Ct.
II. METHODS
A. Chemicals
Chemicals were of the commercially available highest quality and were used without further purification. Chitosan was purchased from Aldrich. The other reagents were purchased from Fluka. Aqueous solutions were prepared using double-distilled deionised water and purged with nitrogen gas prior to each experiment.
B. Apparatus and procedures
A Multi-potentiostat 1030 (CH Instruments- Austin Texas, USA) connected to a desktop computer was used for differential pulse voltammetric (DPV) analysis. DPV parameters for Cu(II) calibration curves were the following: purge time: 5 min, pre-concentration time: 3 min, equilibration time: 15 s, potential scan rate: 10 mV s-1, pulse amplitude: 100 mV and pulse width: 50 ms (phosphate buffer solution, pH 7.0). DPV parameters for cysteine determination were the following: purge time: 5 min under inert atmosphere; potential scan rate: 10 mV s-1; pulse amplitude: 100 mV and pulse width: 50 ms (phosphate buffer solution, pH 7.0).
The three electrode cell assembly used during experiment with cysteine consisted of the CtCu-GC modified electrode, an Ag/AgCl/KCl (3.0 M) reference electrode and a platinum wire counter electrode. All the potentials are reported versus the above specified reference electrode. The experiments were conducted at room temperature (22±2°C) under N2 atmosphere. Calibrations were analyzed by ordinary linear least-square regression and the relevant results (slopes and intercepts) are reported with their confidence interval (P = 95%).
C. Solutions
CuSO4 (1.0×10-2 mol L-1) stock solution was daily prepared. Chitosan stock solutions were prepared by dissolving 0.0163 g chitosan in 10.0 ml of 2.0 M acetic acid solution.
D. Preparation of Ct-GC and CtCu-GC electrodes
Surface of GC electrode was polished with alumina slurry and sonicated with deionised water for 5 min. After sonication, the electrode was rinsed with deionised water and allowed to dry in the air. Then, it was modified by depositing 6.0 µL of chitosan solution with a microsyringe. The Ct-GC electrode was left to dry in air for 30 min. Before use, it was equilibrated for about 10 min in the supporting electrolyte (phosphate buffer) solution and cycled within the -0.2 – 0.8 V potential range. Usually, 50 cycles were sufficient for reaching a constant voltammetric profile.
CtCu-GC electrodes were obtained by electrodepositing Cu(II) at -0.5 V from Cu(II) solution ranging from 3.99×10-6 to 3.91×10-5 M. The Cu(II) content was checked by differential pulse voltammetry. Every experiment was performed by using a newly prepared CtCu-GC electrode. The Cu(II) containing surface film was removed with a wet cloth at the end of each experiment and the electrode was re-polished as above described.
III. RESULTS AND DISCUSSION
At first, experiments were performed for studying the incorporation of Cu(II) in the chitosan film in buffer media (phosphate solution, pH = 7.0) (Martinez-Huitle et al., 2006). Figure 2 shows the differential pulse voltammograms relevant to a chitosan-modified GC electrode after increasing additions of Cu(II) ranging from 3.99×10-6 to 3.91×10-5 M. At higher Cu(II) concentrations, the peak current did not show any further increase. Because of these results, experiments were performed in order to evaluate the capabilities of the CtCu-GC electrode as a cysteine sensor. To this aim, CtCu-GC electrodes were equilibrated with the 3.91×10-5 M Cu(II) concentration (maximum loading), rinsed and immersed in the phosphate buffer, pH 7.
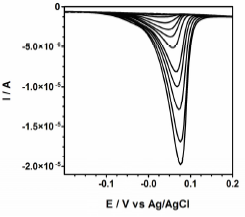
Fig. 2: Voltammograms at Ct-GC, recorded in the buffer solution (pH=7.0) base electrolyte and after equilibration with Cu(II) concentrations ranging from 3.99×10-6 to 3.91×10-5 M. Ct-GC modified electrode as working electrode, a Ag/AgCl/KCl (3.0 M) as reference electrode and a platinum wire as counter electrode. Temperature: 25°C.
On the other hand, CV measurements at Ct-GC electrode were recorded before and after the DPV experiments (Fig. 3). After deposited higher metal Cu ion concentrations, Ct-GC electrode presented a peak response in the investigated potential range after the DPV experiments. These observations suggested that the Ct-modified electrode has a strong affinity through surface coordination between metal ion and Ct film.
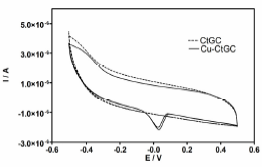
Fig. 3: Cyclic voltammograms achieved before and after DPV experiments at Ct-GC electrodes. Electrochemical behaviour of (-----) modified Ct-GC and (—) CtCu-GC electrodes at supporting electrolyte (buffer solution). Ct-GC modified electrode as working electrode, a Ag/AgCl/KCl (3.0 M) as reference electrode and a platinum wire as counter electrode. Temperature: 25°C.
The coordination is fairly strong with two ligands around each metal ion. These surface complex structures have been already reported by other researchers, in the case of Cu(II) and Pb(II) (see Fig. 1) (Kamiñski and Modrzejewska, 1997; Martinez-Huitle et al., 2006).
In order to show the good linearity during the electrochemical deposition of Cu on Ct film, an example calibration plot relevant from the DPV analysis for Cu deposition (Fig. 2) is shown in Fig. 4a. In this case, DPV measurements were obtained by using the standard addition as method. In addition, the window at the bottom (Fig. 4b) shows that the residuals of the regression are randomly distributed around the zero, allowing a visual verification of the absence of significant non linearity.

Fig. 4: Example of calibration plot relevant to the analysis of Cu (standard additions). The graphic at the bottom displays the residuals.
Some peak current response vs. Cu(II) concentration functional relationships obtained at different Ct-GC electrodes (by using a different number of standard solutions) are reported in Table I. Figure 5 shows an example of responses of such an electrode to increasing cysteine concentrations. As it can be seen, the addition of cysteine produces a decrease of the Cu(II) peak intensity and negligible peak potential shifts. No other peaks were evidenced in the explored potential range, so that it can be argued that the peak current can be only attributed to the reduction of the free Cu(II) in the chitosan film. No peak can be ascribed to any Cu(II)-cysteinate complex. These results allowed to estimate few calibration diagrams of cysteine at different CtCu-GC electrodes, obtained by considering at least thirteen concentrations of cysteine and evaluating the Cu(II) peak intensity as a function of the cysteine concentration.
Table I - Response-concentration functional relationships obtained at different Ct-GC as a function of Cu(II) concentrations. DOF=Degrees of freedom

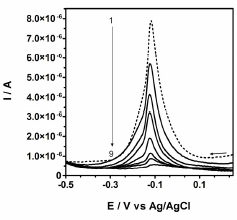
Fig. 5: Voltammograms at CtCu-GC, recorded in a buffer phosphate medium (pH=7.0): Curve 1: recorded in the absence of cysteine; curves 2-8 recorded in the presence of cysteine concentrations ranging from 2.0×10-6 to 5.5×10-5 mol L-1. Ct-GC modified electrode as working electrode, a Ag/AgCl/KCl (3.0 M) as reference electrode and a platinum wire as counter electrode. Temperature: 25°C.
The visual analysis of the regression residuals allowed to estimate a linear range spanning from 2.0×10-6 to 5.5×10-5 mol L-1. After any Cu(II) loading the CtCu-GC electrode, transferred to the pure supporting electrolyte, still exhibited the same peak current, which was unchanged for al least few hours. Few examples of the relevant regression functions are reported in Table II. As it can be seen the determination coefficients are always larger than 0.99.
Table II - Response-concentration functional relationships obtained at different CtCu-GC electrodes as a function of the cysteine concentration. DOF=Degrees of freedom

However, the results in Table II evidence a certain variability of slopes and intercepts. This is not surprising since it is known (Ugo et al., 2001) that responses at polymer modified electrode strongly depend on the actual status of their electrode surface.
On the other hand, a preliminary estimation of the Limit Of Detection, LOD, was also possible by using the approach base on the standard deviation of the regression, sy/x, that is (see reference Miller and Miller (2005) for a recent review on LOD approaches): LOD = (3.3·Sy/x)/b. This approach allows controlling both type I and type II errors at 5% (Miller and Miller, 2005). According to these preliminary results, LOD range from 1.0×10-6 to 1.4×10-6 M. This value seems quite higher than those previously published (Forsman, 1981; Van den Berg et al., 1988); therefore, these results have demonstrated the viability of the application of this sensor for determining cysteine.
III. REMARKS
Further experiments are in progress in order to improve the reproducibility of the CtCu-GC sensor and evaluate its analytical performances. Experiments are being planned to evaluate, for example, different methods for preparing the chitosan surface film and the effects of different Cu(II) loadings. Likely, it should be possible improving (lowering) the LOD. Moreover, experiments will be performed to estimate other figures of merit (e.g. precision, uncertainty of measurement, selectivity, robustness, etc.). The present work could be extremely significant in the neurochemistry field, since future advances will include even smaller microelectrodes for cysteine measurements in the tissues in order of causing less damage to the tissues when implanted.
REFERENCES
1. Agüí, L., C. Peña-Farfal, P. Yáñez-Sedeño and J.M. Pingarrón, "Electrochemical determination of homocysteine at a gold nanoparticle-modified electrode",Talanta, 74, 412-20 (2007). [ Links ]
2. Amarnath, K., V. Amarnath, K. Amarnath, H.L. Valentine and W.M. Valentine, "A specific HPLC-UV method for the determination of cysteine and related aminothiols in biological samples", Talanta, 60, 1229-1238 (2003). [ Links ]
3. Amini, M.K., J.H. Khorasani, S.S. Khaloo and S. Tangestaninejad, "Cobalt(II) salophen-modified carbon-paste electrode for potentiometric and voltammetric determination of cysteine", Anal. Biochem., 320, 32-38 (2003). [ Links ]
4. Brückner, H., M. Langer, M. Lüpke, T. Westhauser and H. Godel, "Liquid chromatographic determination of amino acid enantiomers by derivatization with o-phthaldialdehyde and chiral thiols. Applications with reference to food science", J. Chromatogr. A 697, 229-245 (1995). [ Links ]
5. Elias, R.J., D.J. McClements and E.A. Decker, "Antioxidant activity of cysteine, tryptophan, and methionine residues in continuous phase ß-lactoglobulin in oil-in-water emulsions", J. Agr. Food Chem., 53, 10248-10253 (2005). [ Links ]
6. Forsman, U., "Cathodic stripping voltammetric behaviour of cysteine and cystine in the presence of cupric ions", J. Electroanal. Chem., 122, 215-231 (1981). [ Links ]
7. Forsman, U., "The stripping voltammetmc pattern of cysteine and penicillamine at the mercury electrode in the presence of Cu(II)", J. Electroanal. Chem., 152, 241-254 (1983). [ Links ]
8. Friedman, M., "Improvement in the safety of foods by SH-containing amino acids and peptides. A review", J. Agr. Food Chem., 42, 3-20 (1994). [ Links ]
9. Heyrowský, M., P. Mader, S. Vavricka, V. Veselá and M. Fedurco, "The anodic reactions at mercury electrodes due to cysteine", J. Electroanal. Chem., 430, 103-117 (1997). [ Links ]
10. Jin, W. and Y. Wang, "Determination of cysteine by capillary zone electrophoresis with end-column amperometric detection at a gold/mercury amalgam microelectrode without deoxygenation", J. Chromatogr. A, 769, 307-314 (1997). [ Links ]
11. Kamiñski, W. and Z. Modrzejewska, "Application of Chitosan Membranes in Separation of Heavy Metal Ions", Sep. Sci. Technol., 32, 2659-2668 (1997). [ Links ]
12. Kawasaky, M., T. Kakizaki, W. Hu and K. Hasebe, "Electrode behavior of an osmium-cysteine system on a mercury surface", Anal. Sci., 15, 695-699 (1999). [ Links ]
13. Koh, B.K., M.V. Karwe and K.M. Schaich, "Effects of cysteine on free radical production and protein modification in extruded wheat flour", Cereal Chemistry, 73, 115-122 (1996). [ Links ]
14. Martinez-Huitle, C.A., B. Brunetti and E. Desimoni, "Elettrodi di carbone vetroso modificati con chitosano: comportamento elettroanalitico in funzione del metodo di preparazione", XXII Congresso Nazionale della Società Chimica Italiana, Firenze, 1, 109 (2006). [ Links ]
15. Miller, J.N. and J.C. Miller, Statistics and Chemometrics for Analytical Chemistry. Fifth edition. United Kingdom (2005). [ Links ]
16. Raftery, M.J., U. Justesen, H. Jaeschke and S.J. Gaskell, "Mass spectrometric quantification of cysteine-containing leukotrienes in rat bile using 13C-labeled internal standards", Biol. Mass Spectrometry, 21, 509-516 (1992). [ Links ]
17. Rozhnova, O.I., D.L. Kotova, V.F. Selemenev and T.A. Krysanova, "Spectrophotometric determination of cysteine in aqueous solutions", J. Anal. Chem., 54, 1120-1122 (1999). [ Links ]
18. Schmuhl, R., H.M. Krieg and K. Keizer, "Adsorption of Cu(II) and Cr(VI) ions by chitosan: Kinetics and equilibrium studies", Water SA, 27, 1-7 (2001). [ Links ]
19. Sugawara, K., S. Hoshi, K. Akatsuka and K. Shimazu, "Electrochemical behavior of cysteine at a Nafion®|[cobalt(II) modified electrode]", J. Electroanal. Chem., 414, 253-256 (1996). [ Links ]
20. Tseng, K-S, L-C Chen and K-C Ho, "Amperometric Detection of Cysteine at an In3+ Stabilized Indium Hexacyanoferrate Modified Electrode", Electroanalysis, 18, 1306-1312 (2006). [ Links ]
21. Ugo, P., "Trace iron determination by cyclic and multiple square-wave voltammetry at nafion coated electrodes. Application to pore-water analysis", Electroanalysis, 13, 661-668 (2001). [ Links ]
22. Van den Berg, C.M.G., B.C. Househam and J.P. Riley, "Determination of cystine and cysteine in seawater using cathodic stripping voltammetry in the presence of Cu(II)", J. Electroanal. Chem., 239, 137-148 (1988). [ Links ]
23. Wang, H., W.S. Wang and H.S. Zhang, "Spectrofluorimetic determination of cysteine based on the fluorescence inhibition of Cd(II)-8-hydroxyquinoline-5-sulphonic acid complex by cysteine", Talanta, 53, 1015-1019 (2001). [ Links ]
24. Zaia, D.A.M., K.C.L. Ribas and C.T.B.V. Zaia, "Spectrophotometric determination of cysteine and/or carbocysteine in a mixture of amino acids, shampoo, and pharmaceutical products using p- benzoquinone", Talanta, 50, 1003-1010 (1999). [ Links ]
25. Zeng, H.L., H-F. Li, X. Wang and J-M. Lin, "Development of a gel monolithic column polydimethylsiloxane microfluidic device for rapid electrophoresis separation", Talanta, 69, 226-231 (2006). [ Links ]
26. Zhu, L., Y. Li and G. Zhu, "Electrochemiluminescent determination of L-cysteine with a flow-injection analysis system", Anal. Sci., 19, 575-578 (2003). [ Links ]
Received: November 6, 2008.
Accepted: February 17, 2009.
Recommended by Subject Editor Ricardo Gómez.














