Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Latin American applied research
versión impresa ISSN 0327-0793
Lat. Am. appl. res. vol.40 no.1 Bahía Blanca ene. 2010
ARTICLES
Physicochemical properties and vapor-liquid equilibrium data for steam-distilled lemon essential oil
R.A. Clará, A.C. Gómez Marigliano and H.N. Sólimo
Departamento de Física, Facultad de Ciencias Exactas y Tecnología, Universidad Nacional de Tucumán, Avenida Independencia 1800, 4000
San Miguel de Tucumán, Argentina.
Abstract - Density and refractive index for steam-distilled lemon essential oil were obtained at several temperatures and vapor pressure measurements over the pressure and temperature ranges of P= (2.5 to 80.0) kPa and T = (342.57 to 440.39) K, respectively. Dependence with temperature for these experimental results were fitted to empirical polynomial relations, in order to obtain their coefficients and standard deviations. Calculated values are in good agreement with the experimental ones. The molar enthalpy of vaporization for steam-distilled lemon essential oil was calculated plotting the logarithm of the vapor pressure against the reciprocal temperature. Vapor-liquid equilibrium data of some key components were also obtained over the pressure and temperature ranges of P = (10.0 to 80.0) kPa and T = (374.46 to 440.39) K, respectively. Compositions of both equilibrium phases were determined for these key components by gas chromatography (GC). The essential oil components were characterized by their GC-retention times and the results are compared with gas chromatogram (GC/ MSD) data previously reported in the literature.
Keywords - Steam-Distilled Lemon Essential Oil; Vapor-Liquid Equilibrium; Density; Refractive Index; Vapor Pressure.
I. INTRODUCTION
Tucumán, located in northwest of Argentina, is an important producer of lemon essential oil (Sinclair, 1984). It is mainly obtained by using extractor machines (Bauer et al., 2001; Dewick, 2002; Baser, 1995), which simultaneously extract oil and juice from the lemon fruit. Cold pressed lemon peel oil is a valuable raw material for food, cosmetic, and perfume industries because of its typical citrus flavor. The essential oil is a complex mixture constituted by more than a hundred compounds, such as monoterpenes (> 90 percent of the oil), monoterpene oxygenated derivatives (linalool, citral, esters, etc.), sesquiterpenes (C10 and C15 terpene unsaturated hydrocarbons) and a non-volatile residue constituted by waxes, pigments, coumarins, and psoralens (Braddock, 1999; Hui, 1992). The concentration of these components depends of several factors, such as the fruit variety, processing conditions, weather, soil, degree of maturity of the fruit, etc.
Cold pressed lemon essential oil is a yellow to yellow-greenish colored liquid with a natural fruit aroma characteristic of the lemon from which it is obtained. However, the steam-distilled essential oil is a colorless liquid, which has a similar aroma to the cold pressed oil but practically does not have any non-volatile residue. The lack of color of the steam-distilled essential oil, due to the absence of pigments and coumarins, is one of the most important differences with respect to the cold pressed essential oil, because it leads to very different ultraviolet absorption spectra (Sale, 1953).
Physicochemical properties such as density, refractive index, vapor pressure, and vapor-liquid equilibrium (VLE) data are useful for a full understanding of the thermodynamic properties, as well as for practical chemical engineering purposes.
In this paper we report density ? and refractive index nD at several temperatures, and vapor pressure P data over the pressure range P = (2.5 to 80.0) kPa for steam-distilled lemon essential oil (obtained from the EUREKA lemon variety). Vapor-liquid equilibrium data are also reported over the pressure range P = (10.0 to 80.0) kPa. From these last experimental results, we study how some key lemon oil components (a-thujene, a-pinene, sabinene, ß-pinene, d-limonene, ?-terpinene, neral, and geranial) are fractionated between the liquid and vapor phases. These compounds are very important because they have a significant impact on the top notes of lemon essential oil.
Empiric equations for density, vapor pressure, and refractive index for the sodium D-line of the essential lemon oil as a function of the temperature were developed. These equations are useful for interpolation data within the studied temperature range. Furthermore, the molar enthalpy of vaporization was calculated from the dependence of the logarithm of the vapor pressure with the reciprocal of temperature. All empiric equations were fitted with polynomial equations, in order to obtain their coefficients and standard deviations.
Although the properties of the steam-distilled lemon essential oil could change slightly among different lots as a consequence of changes in the processing conditions, lemon variety, etc., these experimental values can be considered as representative of this lemon oil type. Furthermore, thermodynamic properties of natural products are rather rare in the literature and no experimental values for the studied essential oil could be found.
II. MATERIAL AND METHODS
Steam-distilled lemon essential oil was obtained from the waste filter cake arising from the dewaxing process of cold pressed lemon essential oil (Correa et al., 2008), which was provided by a local factory. On the other hand, d-limonene, a-pinene, ß-pinene, sabinene, ?-terpinene, linalool, and citral (neral + geranial) were supplied by Extrasynthèse (France) with their chromatographic reports. They were used as standard compounds to obtain the corresponding GC-retention time.
Density and refractive index for the sodium D-line, were measured with a vibrating tube densimeter KEM DA-300 with a built-in thermostatic unit accurate to 0.01 K, which allows working over the range T = (277 to 363) K using degassed bidistilled water and dry air as calibrating substances and a Leica AR600 refractometer, respectively. The accuracies were of ± 0.1 kg·m-3 for density and ± 0.00005 for refractive index. On the other hand, vapor pressure and VLE data were obtained with a commercial equipment (Labodest, model 602-S) available from Fischer Technology, Germany, operated as previously reported (Campos et al., 2007). The status of equilibrium is reached by constant recycling of the liquid and condensed vapor phases, and their compositions are measured at stationary conditions. The equilibrium temperatures were measured with a digital temperature logging module tmg, type DTM5080 with a Pt-100 temperature sensor calibrated by the manufacturer with an uncertainty of 0.02 K, whereas total pressure in the still was measured with a precision pressure transmitter Wika, model P-10 with a uncertainty of 0.1 kPa, both connected to the Fischer VLE2+ vacuum and temperature control unit.
Samples of condensed vapor and liquid phases at equilibrium were taken and their compositions were determined by gas chromatography. A Hewlett Packard 6890 gas chromatograph with an automatic injector (Agilent G2613A) directly connected to a ChemStation (HP G2070AA) was used. Quantification was based on the factor responses (RF) of the identified peaks. Good separation of all components was obtained on a 100 m long × 0.25 mm inner diameter × 0.5 µm film thickness crosslinked methyl siloxane capillary column (HP-1 19091Z-530). The used temperature program was as follows: initial temperature T = 363 K for twelve minutes, ramp of 1 K·min-1, final temperature T = 523 K for another ten minutes, and a post run at 573 K for ten minutes, making a total run time of 182 minutes. The nitrogen carrier gas flow was kept constant electronically, working with a split ratio of 20:1 and with the injector maintained at T = 513 K. Detection was conducted by a FID detector at T = 533 K. Three analyses were performed for each sample to obtain a mean mass percent value with repeatability better than one percent.
III. RESULTS AND DISCUSSION
In order to characterize and to clearly define the studied system, a gas chromatogram of the steam-distilled essential lemon oil was obtained. The major constituents, and some minor ones, were identified and quantified based on response factors (R.F.) previously reported (Chamblee et al., 1991). In this work, we assume that these R.F. are the same as ours, since the experimental conditions are similar. The estimate error is ± 0.1 in mass percent.
The identification was carried out by retention times (RT) of the Extrasynthèse chemicals, and also by comparing our chromatogram with that previously obtained by GC/Mass Spectra (Dugo et al., 1999). Since only some key components were studied in our work, Table 1 lists them together with their respective peak area percent, response factors, and concentrations expressed in mass percent.
Table 1. Quantitative data for the studied compounds, along with their response factors (R.F.), and concentrations for steam-distilled essential lemon oil. wi denotes mass fraction of component i.

a From Dugo et al., 1999.
b The difference between this value and 100, correspond to all the other compounds not studied in this work.
In this work, only two types of components included in the steam-distilled essential lemon oil were studied: i) monoterpenes (a-thujene, a-pinene, ß-pinene, sabinene, ?-cymene, d-limonene, and ?-terpinene) and ii) oxygenated terpenes (neral and geranial) which, all together, represent approximately 90 percent of the oil, as can be seen in Table 1.
The measured density ? and refractive index nD are given in Table 2, while vapor pressure data are given in Table 3. Figures 1 and 2 show the experimental refractive index nD and density ? data against temperature, respectively, while Fig. 3 shows the logarithm of the experimental vapor pressure data P against the reciprocal temperature over the pressure and temperature ranges P = (2.5 to 80.0) kPa and T = (342.57 to 440.39) K, respectively, for the steam-distilled lemon essential oil.
Table 2. Experimental refractive index, nD, and density, ?, data at several temperatures, T, for steam-distilled lemon essential oil.

Table 3. Experimental vapor pressure, P, data at several temperatures, T, for steam-distilled lemon essential oil.
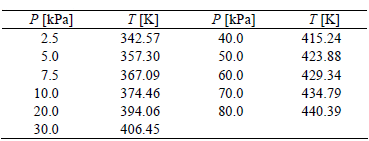

Figure 1. Density ? against temperature T for steam-distilled lemon essential oil. The error bars are smaller than the plotted points.

Figure 2. Refractive index nD against temperature T for steam-distilled lemon essential oil. The error bars are smaller than the plotted points.

Figure 3. Logarithm of the vapor pressure P against the reciprocal temperature T-1 for steam-distilled lemon essential oil. The error bars are smaller than the plotted points.
From these results, empiric equations for density, refractive index for the sodium D-line, and logarithm of the vapor pressure for steam-distilled essential lemon oil as a function of the temperature were developed, as follows:
For density:
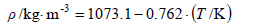 | (1) |
with a standard deviation of ± 0.5 kg·m-3.
For refractive index:
 | (2) |
with a standard deviation of ± 2·10-4.
For the logarithm of the vapor pressure:
 | (3) |
with a standard deviation of ± 0.05. Therefore, P can be calculated from Eq. 3 with a standard deviation of 5 percent.
Differentiating Eq. 3 with respect to T and using the Clausius-Clapeyron equation, the dependence with temperature for the molar enthalpy of vaporization ?HV of the lemon essential oil can be obtained:
 | (4) |
where R is the universal gas constant (= 8.31451 J mol-1·K-1) and T is the absolute temperature in Kelvin. Appling Eq. 4 at 298.15 K, we obtain that ?HV = 54.2 kJ·mol-1, which is higher than the molar enthalpy of vaporization of d-limonene at the same temperature (= 48.1 kJ·mol-1) (Riddick et al. 1986) but, as could be expected, close to it, since the lemon essential oil has 70 mass percent of d-limonene approximately, but also contain another chemical species with higher molar enthalpy of vaporization (oxygenated monoterpenoids and sesquiterpenes compounds). Numerical values of the coefficients were obtained from a least squares analysis of the data. The number of coefficients used for each property was determined as the minimum number needed to adequately represent the data. The significant digits were determined taking into account each experimental error. The standard deviation s, between the experimental and calculated values was defined as:
 | (5) |
where Y represent either ln P, ?, or nD, while N and p are the numbers of experimental points and parameters, respectively.
Vapor-liquid equilibrium (VLE) data over the pressure and temperature ranges P = (10.0 to 80.0) kPa and T = (374.46 to 440.39) K, respectively are given in Table 4. Figures 4-12 show the VLE results for some key components of the steam-distilled lemon essential oil: a-thujene, a-pinene, sabinene, ß-pinene, p-cymene, d-limonene, ?-terpinene, neral, and geranial, respectively. The estimate error is ± 0.1 mass percent.
Table 4. Vapor-liquid equilibrium data of some key components for steam-distilled lemon essential oil over the pressure range P = (10.0 to 80.0) kPa a
aa-thuj., a-pin., sabin., ß-pin., p-cym., limon., ?-terp., ner., and ger. denote a-thujene, a-pinene, sabinene, p-cymene, d-limonene, ?-terpinene, neral, and geranial, respectively.

Figure 4. a-thujene mass percent in: (), vapor phase; ( ), liquid phase against the pressure P over the measured range (10.0 to 80.0) kPa. I, error bars.
), liquid phase against the pressure P over the measured range (10.0 to 80.0) kPa. I, error bars.

Figure 5. a-pinene mass percent in: (), vapor phase; ( ), liquid phase against the pressure P over the measured range (10.0 to 80.0) kPa. I, error bars.
), liquid phase against the pressure P over the measured range (10.0 to 80.0) kPa. I, error bars.

Figure 6. Sabinene mass percent in: (), vapor phase; ( ), liquid phase against the pressure P over the measured range (10.0 to 80.0) kPa. I, error bars.
), liquid phase against the pressure P over the measured range (10.0 to 80.0) kPa. I, error bars.

Figure 7. ß-pinene mass percent in: (), vapor phase; ( ), liquid phase against the pressure P over the measured range (10.0 to 80.0) kPa. I, error bars.
), liquid phase against the pressure P over the measured range (10.0 to 80.0) kPa. I, error bars.

Figure 8. p-cymene mass percent in: (), vapor phase; ( ), liquid phase against the pressure P over the measured range (10.0 to 80.0) kPa. I, error bars.
), liquid phase against the pressure P over the measured range (10.0 to 80.0) kPa. I, error bars.
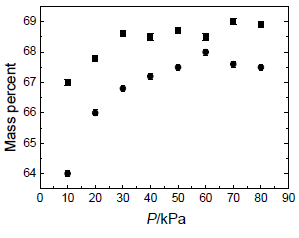
Figure 9. d-limonene mass percent in: (), vapor phase; ( ), liquid phase against the pressure P over the measured range (10.0 to 80.0) kPa.
), liquid phase against the pressure P over the measured range (10.0 to 80.0) kPa.

Figure 10. ?-terpinene mass percent in: (), vapor phase; ( ), liquid phase against the pressure P over the measured range (10.0 to 80.0) kPa. I, error bars.
), liquid phase against the pressure P over the measured range (10.0 to 80.0) kPa. I, error bars.

Figure 11. Neral mass percent in: (), vapor phase; ( ), liquid phase against the pressure P over the measured range (10.0 to 80.0) kPa. I, error bars.
), liquid phase against the pressure P over the measured range (10.0 to 80.0) kPa. I, error bars.

Figure 12. Geranial mass percent in: (), vapor phase; ( ), liquid phase against the pressure P over the measured range (10.0 to 80.0) kPa. I, error bars.
), liquid phase against the pressure P over the measured range (10.0 to 80.0) kPa. I, error bars.
From our experimental results we can conclude that the density and refractive index against the temperature show a linear and quadratic behavior, respectively (see Eqs. 1 and 2). On the other hand, a quadratic relationship was also obtained for the dependence of the logarithm of vapor pressure against temperature (see Eq. 3). Using those relations, the density, refractive index, and vapor pressure for steam-distilled lemon essential oil can be calculated within the temperature and pressure ranges studied. Calculated values are in good agreement with experimental ones.
The molar enthalpy of vaporization was also calculated at 298.15 K and compared with the reported value for d-limonene at the same temperature (Riddick et al., 1986). The calculated value is in agreement with the reported one, since it is slightly greater than that for d-limonene, due to the presence of several minor compounds in the oil with higher molar enthalpy of vaporization.
Vapor-liquid equilibrium data over the pressure and temperature ranges P=(10.0 to 80.0) kPa and T=(374.46 to 440.39) K, respectively for some key components of the steam-distilled lemon essential oil were also obtained. From these results we can conclude that liquid and vapor phases have different concentrations for each component. Therefore, it is possible to increase the concentration of some important commercial components in the lemon essential oil, particularly citral (neral + geranial), as can be seen in Figs. 4-12 and Table 4. These plots show that the vapor phase is richer in a-thujene, a-pinene, sabinene, and ß-pinene than the liquid phase, while p-cymene and d-limonene show similar concentration in both phases. On the other hand, an opposite trend is observed for ?-terpinene, neral, and geranial. Therefore, the deterpenation process of the steam-distilled lemon essential oil is possible because the liquid phase is richer in oxygenated compounds than the liquid one as a consequence of the partial removal of a-thujene, a-pinene, sabinene, and ß-pinene. However, both phases have a similar content of p-cymene and d-limonene, except at P = 10.0 kPa. Therefore, this pressure appears as the better one to make the deterpenation process in the studied pressure range, because the difference between the concentrations of each component in both phases for all the components studied is greater than for the other pressure values.
AKNOWLEDGMENTS
Financial support from the Agencia Nacional de Promoción Científica y Tecnológica and Consejo de Investigaciones de la Universidad Nacional de Tucumán, both of Argentina (Grants: PICTO 2004 N° 633 and CIUNT 26/E417, respectively) is gratefully acknowledged.
REFERENCES
1. Baser, K.H.C., A Manual on Essential Oil Industry, Silva K. T. Ed., UNIDO, Vienna (1995). [ Links ]
2. Bauer, K., D. Garbe and H. Surburg, Common Fragrance and Flavor Materials: Preparation, Properties and Uses, 2nd ed., Wiley-VCH, Weinheim (2001). [ Links ]
3. Braddock, R. J., Handbook of Citrus by-Products and Processing Technology, John Wiley & Sons, Inc. (1999). [ Links ]
4. Campos, V., A.C. Gómez Marigliano and H.N. Sólimo, "Density, viscosity, refractive index, excess molar volume, viscosity and refractive index deviations, and their correlations for (formamide + water) system. Isobaric (vapour + liquid) equilibrium at 2.5 kPa," J. Chem. Eng. Data, 53, 211-216 (2007). [ Links ]
5. Chamblee, T.S., B.C. Clark, G.B. Brewster, T. Radford and G.A. Iacobucci, "Quantitative analysis of the volatile constituents of lemon peel oil. Effects of silica gel chromatography on the composition of its hydrocarbon and oxygenated fractions," J. Agric. Food Chem., 39, 162-169 (1991). [ Links ]
6. Correa, C., M. Gramajo de Doz, C. Bonatti and H. Solimo, "Recovery of lemon essential oil, lemon wax, and diatomaceous earth from the filter cake of the lemon essential oil dewaxing process at pilot-plant scale," Ind. Eng. Chem. Res., 47, 9573-9580 (2008). [ Links ]
7. Dewick, P. M., Medicinal Natural Products: A Biosynthetic Approach, 2nd ed. Wiley, Chichester (2002). [ Links ]
8. Dugo, G., K.D. Bartle, I. Bonaccorsi, M. Catalfamo, A. Cotroneo, P. Dugo, G. Lamonica, H. McNair, L. Mondello, P. Previti, I. Stagno d'Alcontres, A. Trozzi and A. Verzera, "Advanced analytical techniques for the analysis of citrus essential oils," Essenz. Deriv. Agrum., 69, 79-111 (1999). [ Links ]
9. Hui, H.Y., Encyclopedia of Food Science and Technology, John Wiley & Sons Inc. (1992). [ Links ]
10. Riddick, J.A., W.B. Bunger and T.K. Sakano, Organic Solvents. Physical Properties and Methods of Purification, 4th ed., Wiley-Interscience, New York (1986). [ Links ]
11. Sale, J.W., "Analysis of lemon oils", J. Assoc. Offic. Agr. Chem., 36, 112-119 (1953). [ Links ]
12. Sinclair, W.B., The Biochemistry and Physiology of the Lemon and Other Citrus Fruit, University of California, Division of Agriculture and National Resources, California, USA (1984). [ Links ]
Received: September 26, 2008.
Accepted: February 27, 2009.
Recommended by Subject Editor Alcântara Pessôa.














