Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Latin American applied research
versión impresa ISSN 0327-0793
Lat. Am. appl. res. vol.41 no.1 Bahía Blanca ene. 2011
ARTICLES
Production of rigid polyurethane foams from soy-based polyols
A.A. Beltrán and L.A. Boyacá
MSc. Ing. Química, Univ. Nac. de Colombia, Bogotá, Colombia
aabeltrano@unal.edu.co
Dto. de Ing. Química, Univ. Nac. de Colombia, Bogotá, Colombia
laboyacam@unal.edu.co
Abstract - The production of rigid polyurethane foams is made varying the soy-based polyol percentage between 20 and 30% into the formulation. Four blocks of foam are obtained using two oleochemicals polyols from ethanol and ethylenglycol (with funcionalities of 2 and 6, respectively). The foams were characterized by thermal and mechanic tests finding a conductivity value between 0.022-0.026 W/mK and a compressive strength of 15-20 psi, comparables with the commercial specifications and showing the obtained product as appropriate PU foam for industrial applications.
Keywords - Polyurethane; Rigid Foams; Oleochemical Polyester Polyols; Soy-Based Polyols; Ethylene Glycol.
I. INTRODUCTION
Polyurethane (PU) is widely used in various applications as bulk plastics, elastomers, fibers, surface coatings, adhesives, sealants and foams (Latere et al., 2005). PUs are present in different commercial applications such as seating, packaging, footwear, appliances, construction and furniture. Plus, these rank fifth in the production volume of plastics in the world and their consumption is increasing rapidly throughout the world (Tu et al., 2007). PU is currently considered to be one of the most effective materials for insulation, reducing thickness and costs, with a very low conductivity (0,022 W/mK) and a very high ecoeficiency index to save energy (ANPE, 2008). Thus rigid PU foams are used especially in engineering applications such as insulation materials, automotive parts, and structural materials (Narine et al., 2007).
The monomers used to form polyurethanes foams are organic isocyanates and polyols (including polyether and polyester polyols), which are mostly derived from petrochemical refining of crude oil and coals. For rigid PU foam, besides of the common basic component of MDI (polymeric diphenyl methane diisocyanate) other components like chain extender, blowing agent and surfactants are employed to regulate the morphology of the cells.
Polyols can be extracted or synthesized from natural materials such as plants, oils or wood. In the polymer industry, many types of oils (e.g. castor, soybean or rapeseed) have been used as feedstock due to their abundance and economy (Narine et al., 2007). Polyols made from vegetable oils seem to be a good alternative in rigid foam technology in replacement of starting materials for renewable resources. The benefits of this new class of biopolyols are compatibility with hydrocarbon blowing agents, higher hydrofobicity and improved hydrolytic properties of PU foams obtained with a good oxidative stability (Petrović, 2005).
A 100% biobased PUs with acceptable properties are not available because of the lack of satisfactory results on isocyanate synthesis. So far researchers have developed materials containing more than 50% biobased components with thermomechanical properties comparable to petrobased polyurethanes (Latere et al., 2005).
Nonetheless PUs have limited degradability when discarded after use therefore can be an environmental problem. Along with the increasing of % biopolyol in the foam formulation, it could be spliced hydrophilic chains into the backbone of the triglycerides compounds (due the inner hydrophobicity of PU foams, even those based on vegetable oils). The use of hydrophilic polyols (such as ethylene glycol) lends to increase the degradation rate of the foam, because it entails a rise in polymer permeability, and it has been demonstrated successfully by some authors (Yeganeh and Hojati-Talemie, 2007).
Besides, the use of ethylene glycol in PU foam has a good economic advantage (667 USD/ton) compared to other alternative raw materials like propylenglycol (1000 USD/ton) or pentaeritritol (1278 USD/ton) (Bozell., 2004).
II. METHODS
A. Materials
Polymeric MDI (PAPI 27 from Dow Chemical) was used as isocyanate. For the B side, it was made a mix of polyols that constitutes 100 parts (pph) for the formulation of the rest of compounds: catalysts (Polycat 8, Niax A-1 and Curithane 206), surfactant (Tegostab B-8427) and HCHC-141B as a foaming agent, all provided by Dow Chemical. The polyol mix included Voranol 446, Voranol 640 and soy-based polyols (hydroxyl numbers of 120 from ethanol and 331 mg KOH/g from ethylene glycol). These oleochemical polyols were prepared through an in situ epoxidation of the soybean oil with per acetic acid using a homogeneous catalyst (Boyacá and Beltrán, 2009).
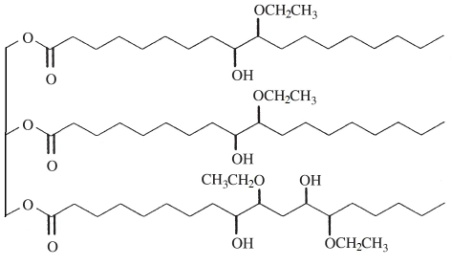
Figure 1. Chemical structure of a soybean oil based polyol from ethylene glycol.
B. Procedures
The foam preparation required a good mixing between two parts, technically known as A (isocyanate) and B sides (polyol and the others). A typical rigid foam formulation is shown in Table 1, with a total approximate weight of 180 g of B side, used later for reactivity tests and foam production. The soybean polyol percentage in the mix was varied between 20 and 30% for a total of 4 blocks of rigid foam, two for each type of oleochemical polyol. For the production of the rigid blocks was used an stainless steel mold (Fig. 2a) with 30cm x 30cm x 7.5cm dimensions, preheated in an oven at 54ºC.
Table 1. Typical formulations for the production of rigid foams.


Figure 2. a. Mold for the production of the blocks, b. Determination of gel time and, c. free tack time.
For each B side formulation, preliminary tests were made to know the behavior and reactivity of the foam, especially the growth rate through the determination of empirical parameters as cream time (visible increase of foam volume), gel time (indicating a transition of the mix from liquid to solid state) and free tack time, for a mixing time of 10 seconds for every tests.
Verified the reactivity of the formulated B side, the both sides were mixed in specific proportions (Table 2), agitated during 10 seconds, and then the mold was filled with the mixture and closed during a foaming time of five minutes (based on reactivity tests). After that the foam was unmolded, measured for density estimation and cut it into appropriate pieces for the measure of thermal and mechanical properties (Fig. 3).
Table 2. Weights of A and B sides added for foaming.
Figure 3. Procedure for preparation and characterization of PU rigid foams (Block 1).
C. Analytical Determinations
The rigid foams were evaluated through the determination of their conductivity and compressive strength, according with the densities obtained (Table 3). For the conductivity measure (ASTM C177-04) it was cut into pieces of 20cm x 20cm x 1.0 inch, two specimens for each block produced and all were analyzed in an Anacon 8, Thermal Conductivity Analyzer during 30 minutes until reach a constant value. The temperatures used were 50 and 100ºC in cold and hot plates and the analysis was performed 24 hours after the foams production. For compressive strength determination (ASTM D1621-04a) pieces of 2x2x1 inches were cut, 4 specimens for each block and all were analyzed in the universal testing machine SYNTECH® 2W until a deformation of 10% (velocity displacement: 1,2 mm/min, temperature: 23 ± 2ºC, relative humidity: 50% ±0.5).
Table 3. Densities of PU foams.

Moreover, foam samples were analyzed trough scanning electronic spectroscopy (SEM) using a FEI QUANTA 2000 device, with acceleration between 500v and 30 kV, using a low vacuum operation mode (1-4 torr) with nitrogen as auxiliary gas. The metallization of each sample was made with a sputter SDC-050, Balzers trademark, in medium vacuum (<2-10 torr) with argon over a Au-Pd plate, films with +/-5 nm of thickness were obtained over the foam samples.
III. RESULTS AND ANALYSIS
A. Reactivity tests
Although, these times are not always reported by the authors, these tests are temporary but important information that helps to know the behavior the formulations and calculate the required weights of each substance in the foam production to assure a complete filled mold. The gel times obtained (Table 4) are quite lower than others reported for soybean polyols (4 min, 40°C) at lower temperatures (Guo et al., 2006) indicating a good foam velocity. Besides, the gel time can be used as an accurate parameter for comparison between foaming velocities (Kapps, 2004). Thus, the ethylene glycol soy-based polyol is more reactive due its average gel time (50 s) lower compared with the ethanol-based polyol (71 s), with a better free tack time (approx. 30 s of difference).
Table 4. Reactivity tests for rigid foams (T=20°C).

B. Thermal and mechanical properties
For each specimen, the universal testing machine provide a curve of load vs elongation (Figure 4), where the maximum load value (Y) can be divided by the area of each sample (4 inches2) to obtained the reported value (an arithmetic average between the 4 specimens). Table 5 shows the compressive strength and conductivity values for all the blocks produced.

Figure 4. Curve of compressive strength test (Block 1).
Table 5. Properties for obtained PU foams.

The principal use for rigid PU foams is thermal insulation, which needs foam densities between 30-100 kg/m3 (Buschkamp, 2001), especially values lowers than 50 kg/m3, depending the final use (Era Polymers, 2005). For densities of 30 kg/m3, typical values of compressive strength are in 100-150 kPa and, with an R value (inverse of K factor or conductivity) between 40 and 60 mK/W (Oertel et al., 1994). The properties obtained for all blocks (Table 5) are into the expected ranges with R values of 37.9-45.2 mK/W and compressive strength of 103.5-141.3 kPa. Then, these results validate the use of the produced foams (and its formulations) in the polymeric industry, especially for insulation in refrigeration and cooling appliances.
Table 5 presents the properties of differents PU foams for densities between 30-70 kg/m3. The characteristics of the obtained foams are comparables with the reported by commercial ones, although the kind of used polyol are not reported. The values obtained are even higher compared with similar studies using soybean oil with a decrease of 15-22% in the conductivity for foams with the same density (Table 6). Due lower K value represent a better insulation material, Guo et al. (2000) show the importance of measure this property properly, obtaining a K factor of 0.020 W/m in a test performed 24 hours after the foam production, value that could increase in time and be wrong reported if there is a delay. Nonetheless, they obtained between 12 and 23% less resistance to compression for soybean polyols.
Table 6. Properties of commercial rigid foams of PU.
C. Evaluation of the foam formulation
The formulation chosen was appropriate for the production of these rigid foams taking into account the good miscibility between components and the cream and gel times obtained in the reactivity tests.
The water content can interfere with the analysis of the obtained properties, with concentrations of 4 and 5.5 pph for ethylene glycol (20 and 30%) and 2 and 2.7 pph for ethanol based polyols; typical formulations includes lower concentrations (3.0 pph) even when water is used as a foaming agent (Tu et al., 2007). Other studies reveal an increasing of resistance with higher water concentrations (Guo et al., 2000), it is known that water can react with isocyanate to form urea structures that provide rigidity to the polymeric structure. But the water, as a foaming agent, increase the cells size and make thinner walls; that leads in a decrease in mechanical properties, a blatant phenomenon in Block 4 which presents the lower compressive strength (103.5 kPa). It could be improved using more surfactant although according to Guo et al. (2000) 2 pph is reported as the optimum value.
In the other hand, it was expected that the comparison between blocks 1 and 3 (made with 20% of oleochemical polyol) made evident a higher compressive strength using a polyol with higher hydroxyl number (Tu et al., 2007) due the OH groups consume more quantity of isocyanate (which generate more cross linking) increasing the rigidity of the matrix.
The results show (Table 5) more resistance using ethanol-based polyol due the higher water content for block 3, analyzed before. Besides, the compressive strength is better for blocks 1 and 2 than 3 and 4 (made with ethylene glycol-based polyol) due their densities values. Conductivity value is also affected by water content. Although water reaction generates the production of carbon dioxide (decreasing the K value), the foam expansion could lead in more open cells, increasing air convection (Tu et al., 2007). Then, block 4 (made with 30% of ethylene glycol-based polyol) shows the highest value (0.026 w/mK).
Finally, it is usually expected that soybean-based polyols presented lower yield during polymerization since they do not present a good hydroxyl number to allow the required cross linking degree for production of PU rigid foam (Guo et al., 2000), where at least a functionality of 3 is used into these formulations. Therefore, in the case of ethylene glycol, it could be increased the oleochemical polyol percentage to 50% or even 100% of petrochemical polyols replacement as reported by other authors (Table 7) with similar formulations.
Table 7. Polyols obtained from soybean oil and its use on a rigid foam formulation
D. Anisotropy
During the expansion phase, the foam unfurl a flow movement in which cells bother each other adopting an extended and oblong shape (Kapps, 2004). Thus, the diameter in the flow direction is higher than in a perpendicular way, generating cell orientation (Fig. 5) that remain in many cases, even after finished the reticulation phase. This phenomenon is clearly evident using ethanol-based polyol at 30% (Fig. 5b) which presents the lower compressive strength for this kind of polyol and the biggest cell size, compare with a less percentage (20%, Fig. 5a). Then, according with the cell size, the block 1 (20% of ethanol-based polyol) is better presenting a decrease of 10% in its conductivity value.






Figura 5. SEM photographies for rigid foams: block 1 (a and c), block 2 (b), block 3 (d), block 4 (e and f).
For ethylene glycol-based polyol, the use of a lower concentration (20%) produced a more uniform distribution (Fig. 5d) and smaller cell sizes than using 30% for this oleochemical polyol (Fig. 5e), but an anisotropic behavior is not evident in any case. Besides, a high percentage can increase the K value in 20%, diminishing its insulation efficiency. Finally, it could be clearly observed a thicker wall for the foam with ethanol-based polyol at 20% (Fig. 5c) compared with a ethylene glycol-based foam (30%, Fig. 5f) which explain the difference between the compressive strength for block 1 (141.3 KPa) and block 4 (103.5 KPa).
IV. CONCLUSIONS
Two soybean-based polyols were used satisfactory in the production of PU foams with appropriate densities for insulation applications. The thermal and mechanical properties of the obtained foams are comparables with those ones offer commercially and reported by others researchers. Although an anisotropic behavior is evident with ethanol-based polyol and its lower functionality, both polyols are suitable of being used in rigid foams. The % oleochemical polyol can be increase diminishing the water content into the foam formulation.
REFERENCES
1. ANPE, "Poliuretano y medio ambiente: fare di più e meglio con meno," Associazione Nazionale Poliuretano Espanso rigido, www.poliuretano.it (2008). [ Links ]
2. Balmoral Offshore Engineering, Technical Information, Balmoral Comtec Ltda, http://www.balmoral-group.com/boe/boe-7-tech-info.aspx (2008). [ Links ]
3. Beltrán A.A. and Boyacá LA., "Preparation of oleochemical polyols derived from soybean oil," Latin American Applied Research, Vol. 41, No. 1, pp- 69-74, (2011). [ Links ]
4. Bozell, J. Oleochemicals as a feedstock for the biorefinery: high value products from fats and oils, Report of National Renewable Energy Laboratory (2004). [ Links ]
5. Buschkamp, S., Rigid polyurethane foam: production - properties - uses, Polyurethanes Business Group, Technical information, 17, http://pu.bayer.de/ (2001). [ Links ]
6. Endres, E., Wärmedämmstoffe aus Polyurethan-Hartschaum, Industrieverband Polyurethan-Hartschaum, IVPU http://www.daemmt-besser.de/ (2008). [ Links ]
7. Era Polymers Pty Ltda, www.erapol.com.au/ (2005). [ Links ]
8. Guo, A., I. Javni and Z. Petrovic, "Rigid polyurethane foams based on soybean oil," Journal of Applied Polymer Science, 77, 467-473 (2000). [ Links ]
9. Guo, A., W. Zhang and Z.S. Pretrovic, "Structure-property relationships in polyurethanes derived from soybean oil," Journal of Material Science, 41, 4914-4920 (2006). [ Links ]
10. Huntsman International LLC, www.huntsman.com/pu/ (2008). [ Links ]
11. Kapps, M., "Fabricación de espuma rígida de poliuretano (PUR)," Bayer Material Science, Technical information: Insultation, File No. PU21012-0409es http://pu.bayer.de/ (2004). [ Links ]
12. Latere J.P., A.K. Mohanty, M. Misra and L.T. Drzal, "Biobased polyurethanes and their composites: present status and future perspective", Natural fibers, biopolymers and biocomposites (Ed. Mohanty). CRC Press, U.S.A. (2005). [ Links ]
13. Narine, S., X. Kong, L. Bouzidi and P. Sporns, "Physical properties of polyurethanes produced from polyols from seed oils: II. Foams", Journal of The American Oil Chemistry Society, 84, 65-72 (2007). [ Links ]
14. Oertel G., Polyurethane Handbook, Hanser Publishers, 2nd edition. Munich (1994). [ Links ]
15. Petrovic Z.S. "Polyurethanes", The Handbook of Polymer Synthesis (Kriecheldorf H.R.), Marcel Dekker, Inc., New York (2005). [ Links ]
16. Tu, Y.-C., P. Kiatsimkul, G. Suppes and F.-H. Hsieh, "Physical properties of water-blown rigid polyurethane foams from vegetable oil-based polyols," J. of Applied Polymer Science, 105, 453-459 (2007). [ Links ]
17. Vilar, W., Química e tecnologia dos poliuretanos, Ed. Vilar Consultoria, 3ra ed., http://www.poliuretanos.com.br/ (2008). [ Links ]
18. Welte, R., "Rigid polyurethane foam for pipe insulation," Bayer Material Science, Technical information: Insulation, File No. PU21018-0410en In: http://pu.bayer.de/ (2004). [ Links ]
19. Yeganeh H. and P. Hojati-Talemie, "Preparation and properties of novel biodegradable polyurethane networks based on castor oil and poly(ethylene glycol)," Polymer Degradation and Stability, 92, 480-489 (2007). [ Links ]
Received: August 25, 2009.
Accepted: January 25, 2010.
Recommended by Subject Editor Ricardo Gómez.














