Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Latin American applied research
versión impresa ISSN 0327-0793
Lat. Am. appl. res. vol.41 no.3 Bahía Blanca jul. 2011
Separation efficiency in a sieve plates extractor
M.M.P. Gambarra, C.C. Lima and L.M.N. Gois
Chemical Engineering Department of Federal University of Bahia
R. Aristides Novis 2. Zip Code: 40210-630. Federação, Salvador-Bahia-Brazil.
Abstract - This work analyzes the separation efficiency data involving mass transfer between two immiscible liquid phases in a bench lab extractor without mechanical agitation. The experimental apparatus consisted in a cylindrical glass column containing perforated plates, operating in countercurrent mode. The experimental runs were developed by using butane-acetic acid-water system, following a factorial experimental design involving the variables: dispersed phase velocity, the continuous phase velocity and the number of stages (two and four plates). The collected acid acetic raffinate and extract was analysed by titration with sodium hydroxide. Separation efficiencies were estimated through Murphree (1925) and Kawase (1990) models. The results showed that the higher was the dispersed flow, higher was the separation efficiency. An opposite behavior was observed for the continuous phase flow effect. In relation to the number of plates, higher efficiencies were achieved for 4 plates arrangement. The results provided empirical equations to predict the extraction efficiency, which exhibited good correlation with experimental data.
Keywords - Liquid-Liquid Extraction; Efficiency; Mass Transfer.
I. INTRODUCTION
In recent years the knowledge about separation efficiency in liquid-liquid extraction columns has notably increased, once it is directly connected to mass transfer between the liquid phases present in the mixture. According to Tudose and Apreotesei (2001) and Lisa et al. (2003), the solute transfer between two immiscible liquids is still a complex phenomenological process. However, its applications, mainly in purification processes, are of great relevance to chemical industries and various types of solvent extraction contactors, including spray and packed columns, have been used for a range of applications in the hydrometallurgical, pharmaceutical and petrochemical industries form many years.
According to Stella et al. (2008) the optimal design of a solvent extraction column involves maximizing the performance by increasing the interfacial area for mass transfer, mass transfer coefficient and concentration driving force. As the interfacial area is dependent on the dispersed phase droplet size and holdup in the column, it is important to accurately predict and optimize the hydrodynamic characteristics.
The performance of an extraction unit operated continuously is also dependent on the amount of solvent present in extractor. If the solvent quantities are high compared to the feed, the solute concentration gradients are favorable to mass transfer (Zuniga et al., 2006).
Jahya et al. (2005), Treyball (1980), Stella et al. (2006) among others evaluated the performance of extraction columns based on data from axial dispersion, holdup, NUT, NETS, etc. However there are few studies in the literature in which the performance of the producers is measured through data separation efficiency
Góis et al. (1999), for example, reported that mass transfer between liquids phases in an extraction column depends, among other factors, on the interfacial contact area between continuous and dispersed phases, and its extension can be evaluated through the separation efficiency measurement.
About efficiency in extraction columns, the literature shows that efficiencies can be evaluated by Murphree's efficiency, where actual exit concentrations are compared with thermodynamic equilibrium conditions. Murphree's efficiency is expressed by
 | (1) |
Another model, also very used to calculate the separation efficiency in extraction columns, was proposed by Kawase (1990). This model considers the efficiency as a solute recuperation ratio in the extract current, as described by
 | (2) |
The exact meaning of the variables in equations (1) and (2) is described in the nomenclature. They represent the concentrations of solute at the top of the column feed (xf), the concentration of the raffinate output (xr) from the base and the equilibrium concentration equivalent for the raffinate 
Therefore, the main objective of the present work was to study the influence of operational and geometrical parameters on the performance of a perforated plate column, using separation efficiency parameters calculated by Murphree (1925) and Kawase (1990) models. The factors whose effects were evaluated were continuous and dispersed phases flows and the number of stages, associated to the column height. Empirical correlations to predict the extraction efficiency based on the models discussed above were also proposed.
II. EXPERIMENTAL APPARATUS
The extraction column used in the present work consisted of a glass tube with 0,0923 m of internal diameter and heights ranging from 0,70 to 0,90 m, corresponding to 2 or 4 stages. The stages were divided by aluminum perforated plates with 14.32% of free area, each one having 65 perforations of 5,5 x10-3 m in diameter. Figure 1 shows a typical experimental setup. The studied system consisted of n-butyl alcohol (dispersed phase), fed at the bottom to move upwards in countercurrent with an aqueous 0.1 M acetic acid solution (the continuous phase). As the dispersed phase (organic) is less dense than the continuous phase (aqueous) is established countercurrent contacting between the two phases inside the column. The plates in turn serve as flow distributors. They make the net current in small circular droplets that cross vertically at each stage of the column. With this crossing is established intimate contact between the two phases and, consequently, the mass transfer of solute (acetic acid) in the aqueous phase to the organic phase. Table 1 lists the fluid-phase system physicochemical data and Table 2 presents the range of variables investigated. All the experiments were conducted at local room temperature (301 - 303K).
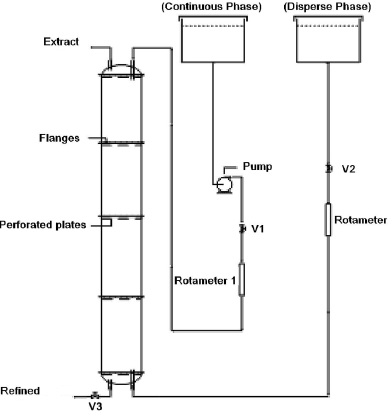
Figure 1 - Schematic diagram of the experimental setup
Table 1. Physicochemical properties of system

Table 2. Ranges of investigated variables
In order to evaluate the efficiency, acetic acid concentrations were measured in the feed current (xf) and in the purified liquid current (xr) by titrating the collected samples against a standard 0.1 M sodium hydroxide solution. Equilibrium data were obtained according to Sorensen and Arlt (1980).
III. RESULTS AND DISCUSSION
The experimental results were expressed in terms of efficiency versus dispersed phase velocity, as shown in Figures 2 to 9. The results were analyzed by taking in account the effects of operational and geometrical factors (continuous and dispersed phases velocity and number of stages) on the column efficiency. These effects were measured in terms of Murphree (1925) and Kawase (1990) efficiency indexes, as discussed below.
A. Effect of continuous and dispersed phases flows
Figures 2 and 3 show, respectively, how Murphree's and Kawase's efficiencies are influenced by the continuous phase flow in a 4 stages experimental setup. On the other hand, Figures 4 and 5 represent a similar analysis related to the experiments realized in a 2 stages experimental arrangement. Both set of situations reveal that the increase of dispersed phase flow increases Murphree and Kawase separation efficiencies. This behavior was already expected once the dispersed phase is the solvent (n-butyl alcohol) whose flowrate influences positively solute recovering (acetic acid)
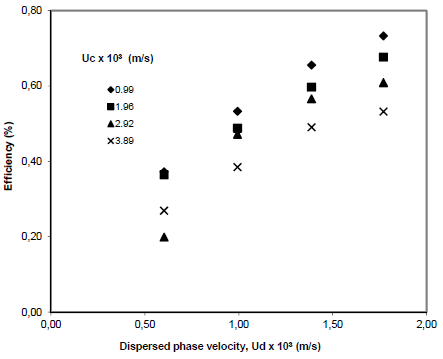
Figure 2 -Effect of disperse phase flow on the separation efficiency (Murphree (1925) model) in a four stages column.

Figure 3 - Effect of dispersed phase flow on the separation efficiency (Kawase (1990) model) in a four stages column.

Figure 4 -Effect of the dispersed phase flow in the separation efficiency (Murphree (1925) model) in a two stages column.

Figure 5 -Effect of the dispersed phase flow in the separation efficiency (Kawase (1990) model) in a two stages column.
B. Comparison between efficiency models
Another important conclusion can be taken by comparing the efficiencies data obtained through Murphree's and Kawase's models. Murphree's efficiencies were greater than Kawase's efficiencies to all respective experimental situations. These results are in disagreement with Coimbra (1991), that studied the extraction of lactic acid by using a rotating discs extractor. According to Coimbra (1991), the efficiency values predicted by the author using Kawase's model were greater than the ones obtained by Murphree (1925) model. In contrast, the present work showed that the efficiency values achieved through Murphree(1925) model were far better than the efficiency values estimated by the use of Kawase (1990) model, as depicted in the Figures 6 to 9. This tendency can be associated to the fact that Murphree's efficiency is related to the thermodynamic equilibrium data, while Kawase's efficiency is only a solute recuperation index.

Figure 6. Comparison between Murphree (1925) and Kawase (1990) models for the separation efficiency in a 4 stages column to continuous phase velocity 9.97 x 10-3 (m/s).

Figure 7. Comparison between Murphree (1925) and Kawase (1990) models for the separation efficiency in a 4 stages column to continuous phase velocity of 3.89 x 10-3 (m/s)

Figure 8. Comparison between Murphree (1925) and Kawase (1990) models for the separation efficiency in 2 stages column to continuous phase velocity of 9.97 x 103 (m/s)

Figure 9. Comparison between the Murphree (1925) and Kawase (1990) models for the separation efficiency in a 2 stages column to continuous phase velocity of 3.89 x 10-3 m/s.
IV. DIMENSIONAL ANALYSIS AND EMPYRICAL EQUATIONS
The dimensional analysis presented below was based on Buckingham's p theorem that consists in an appropriate combination of the variables that exert influences on the phenomenon, generating dimensionless groups. From the Murphree (1925) model and carrying out the proper algebraic treatment discussed above, the following empirical equation was adjusted:
 | (3) |
By applying a similar treatment to the Kawase (1990) model, it was obtained the empirical Eq. (4). It is important to detach that the exponent values in Eqs. (3) and (4) were determined numerically through the use of least square.
 | (4) |
Figures 10 and 11 show the comparisons between experimental and calculated efficiencies values obtained by the use of the empirical correlations (Eqs. (3) and (4)).

Figure 10 - Comparison between experimental e predicted separation efficiencies based on Murphree (1925) model

Figure 11 - Comparison between experimental and predicted separation efficiencies based on Kawase (1990) model.
Figure 10 shows better agreement between experimental and theoretical efficiency values than Fig. 11. The average error produced by the use of Murphree (1925) model was 13.26% against 20.64% obtained by the correlation using Kawase (1990) model, what indicates that Eq. (3) is the best choice to predict the separation efficiency in the system studied. So, in this case, it can be seen a true superiority of Murphree (1925) model over Kawase (1990) model with the purpose of estimating acetic acid extraction efficiency, using n-butyl alcohol as solvent, in a perforated plate column.
VI. CONCLUSIONS
The extractor of perforated plates used in the experiments showed good performance in terms of hydrodynamic behavior. The values of separation efficiency were obtained in the range of 40-60% (Model Murphree, 1925) which indicates good results in the operation of equipment for the system n-butyl alcohol -acetic acid-water.
The dispersed phase velocity has strong influence on the column performance, showing approximately a linear dependence on this parameter. On the other hands, given a certain dispersed phase flow, the increase of continuous phase flow generally caused an efficiency decreasing.
This reduction is justified considering that the increased flow of the continuous phase causes a reduction in the relative velocity between the two phases. Low relative velocities have lower interfacial contact areas and consequently lower efficiency is obtained.
The column size, expressed in terms of the number of stages, also exerted a positive influence on the extraction efficiency. Higher efficiency values were observed for 4 stages experimental arrangements, because the contact time follows the same tendency. These conclusions are in agreement with Sá and Góis (2005) that studied the dispersed phase holdup. It was reported that the holdup of dispersed phase increases with the number of plates, improving the separation efficiency.
Finally, it was observed that Murphree (1925) model provided higher efficiency values in comparison with Kawase (1990) model. Besides, the results predicted by the Murphree type correlation (Eq. (3)) are more compatible with the experimental values.
NOMENCLATURE
xf -concentration of acetic acid in the feed, mol/m3)
xr - concentration of acetic acid in raffinate, mol/m3
xr* - concentration of acetic acid in equilibrium conditions (kg/m3)
Qc - Continuous phase volumetric flow (m3/s)
Qd - Disperse phase volumetric flow (m3/s)
L - Column length (m) D - Column internal diameter (m)
uc- Continuous phase velocity (m/s)
ud- Dispersed phase velocity (m/s)
Re - Reynolds' number (dimensionless)
g - Gravity acceleration (m/s2)
Greek Letters:
λ- Separation efficiency (%)
ρ-Density (kg/m3)
μ- Viscosity (kg/m.s)
REFERENCES
1. Coimbra, J., Análise da eficiência de extração em colunas de disco rotativos na purificação do ácido lático, Dissertação de Mestrado, UNICAMP, Campinas, Brazil (1991). [ Links ]
2. Góis, L.M.N., E.B. Tambourgi, J.A.F.R. Pereira and J.J.P. Marques, "Desempenho de um Extrator de Pratos Pulsantes.," Ciência & Engenharia, 8, 53-59 (1999). [ Links ]
3. Jahya, A.B, H.R.C. Pratt and W.S. Geoffrey, "Comparison of the Performance of a Pulsed Disc and Doughnut Column with a Pulsed Sieve Plate Liquid Extraction Column," Solvent Extraction and Ion Exchange, 23, 307-317 (2005). [ Links ]
4. Kawase, Y., "Dispersed-Phase Holdup and Mass Transfer in a Rotating Disc Contactor with Perforated Skirts," J. Chem. Tech. Biotechnol., 48, 247-260 (1990). [ Links ]
5. Lisa, G.A.; R.Z. Tudose and H. Kadi, "Mass transfer resistance in liquid-liquid extraction with individual phase mixing," Chem. Eng. Process., 42, 909-916 (2003. [ Links ]).
6. Murphree, E.V., "Rectifying Column Calculations," Ind. Eng. Chem., 17, 960-964 (1925). [ Links ]
7. Sá, R.M. and L.M.N. Góis, Estudo da Retenção da Fase Dispersa em Colunas de Extração Líquido-Líquido, Dissertação de Mestrado, UFBA, Salvador, Bahia, Brazil (2005). [ Links ]
8. Sorensen, J.M. and W. Arlt, "Liquid-Liquid Equilibrium Data Collection: Ternary Systems," Chemistry data series (Dechema), V, 227 (1980). [ Links ]
9. Stella, A., H.R.C. Pratt, K.H. Mensforth, G.W. Stevens and T. Bowser, "Backmixing in Karr Reciprocating-Plate Extraction Columns," Ind. Eng. Chem. Res., 45, 6555-6562 (2006). [ Links ]
10. Stella, A., K.H. Mensforth, T. Bowser, G.W. Stevens and H.R.C. Pratt, "Mass Transfer Performance in Karr Reciprocating Plate Extraction Columns," Ind. Eng. Chem. Res. , 47, 3996-4007 (2008). [ Links ]
11. Treyball, E.R., Mass transfer operation, McGraw-Hill (1980). [ Links ]
12. Tudose, R.Z. and G. Apreotesei, "Mass transfer coefficients in liquid-liquid extraction," Chem. Eng. Process., 40, 477-485 (2001). [ Links ]
13. Zuniga, G.D.A, J.S.R Coimbra, L.A. Minim and E.G. Rojas E.G., "Dispersed phase hold-up in a Graesser raining bucket contactor using aqueous two-phase systems," J. Food Eng., 72, 302-309 (2006). [ Links ]
Received: August 7, 2009
Accepted: March 30, 2010
Recommended by Subject Editor: Orlando Alfano














