Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Latin American applied research
Print version ISSN 0327-0793
Lat. Am. appl. res. vol.41 no.4 Bahía Blanca Oct. 2011
ARTICLES
Influence of catalyst treatments on the decomposition of hydrogen peroxide on supported palladium catalysts
F.A.P. Voll, F. Palú and J.B.O. Santos
Centro de Engenharias e Ciências Exatas, Universidade Estadual do Oeste do Paraná,
Rua da Faculdade, 645, CEP 85903-000,Toledo, Paraná, Brazil.
joaobatista35@yahoo.com.br
Abstract The decomposition of hydrogen peroxide was studied on Pd catalysts between 293 K and 313 K. The apparent activation energy value was calculated for all the catalysts and it varied between 27 and 55 kJ/mol. For all the catalysts tested, treatment with hydrogen resulted in a significant increase in the reaction rate, as well as a decrease in the apparent activation energy for the reaction. All the reactions were well represented by a first order rate law with respect to H2O2. The effect of the treatment with H2O2 was studied in successive reactions without catalyst exchange. A small deactivation was observed in the 5.0% Pd/AC and 0.5% Pd/AC catalysts after four H2O2 decomposition reactions.
Keywords Hydrogen peroxide; Palladium Catalyst; Decomposition; Kinetics.
I. INTRODUCTION
Hydrogen peroxide, H2O2, has been used in several applications, such as wastewater treatment (Pignatello, 1992; Lin et al., 1998; Perathoner and Centi, 2005; Dantas et al., 2006; Zazo et al., 2006), as propellant for liquid propellant engines (Sisco et al., 2005), in bleaching paper pulp (Lopez et al., 2003; Wojciak et al., 2007), and in the food and pharmaceutical industries to clean and disinfect packages (Simmons et al., 1997; Verce et al., 2008). In general, the efficiency of these applications depends on the decomposition of H2O2 in homogeneous or heterogeneous mediums. The iron oxide catalyst has been extensively studied in wastewater treatment perhaps due to its low cost (Chou and Huang, 1999; Huang et al., 2001; Hermanek et al., 2007). However, other catalysts have been studied for improving the catalytic activity in those processes (Ariafard et al., 2003; Petlicki et al., 2005; Shivankar and Thakkar, 2005).
The production of H2O2 from hydrogen and oxygen is an environmentally friendly process of great practical importance. Palladium, Pd, is the catalyst most studied for this reaction (Choudhary et al., 2001; Landon et al., 2003;Voloshin et al., 2008; Choudhary and Jana 2008; 2009), but the decomposition of H2O2 on the Pd catalyst is a parallel reaction that decreases the selectivity for H2O2 and, thus, the production of H2O2 becomes inefficient. The H2O2 decomposition on Pd catalyst has been studied by Choudhary et al. (2002, 2006, 2007) under different conditions in order to identify factors that affect the H2O2 decomposition on Pd catalyst. They found that the decomposition of H2O2 decreased with the oxidation of Pd to PdO. In addition, Choudhary et al. (2007) observed that the presence of halide anions (e.g. Cl-, Br-, I-) in the catalyst or in the acidic medium decreased the decomposition of H2O2.
The catalytic decomposition of H2O2 forms water and gaseous oxygen in an exothermic reaction. This reaction occurs on heterogeneous, homogeneous, and enzymatic catalysts but heterogeneous catalysts present advantages such as easy separation of the catalyst, and the catalyst can be used in neutral pH. The kinetics of H2O2 decomposition depends on pH, the initial concentration of H2O2, the reaction temperature, and the catalyst. For example, Gurol and Lin (2002) found that the decomposition rate of H2O2 was proportional to the iron oxide concentration. The H2O2 decomposition decreased when this reaction was carried out in an acidic medium (Choudhary and Gaikwad, 2003). The authors suggested that the decrease in H2O2 decomposition was due to the presence of H+ at higher concentrations. The H2O2 decomposition rate was found to be a first order reaction in relation to H2O2 concentration (Oliveira et al., 1998; Choudhary and Gaikwad, 2003) but another expression describing the H2O2 decomposition was reported by Voloshin et al. (2008). The effect of pH on the decomposition of H2O2 on Co(II) acetylacetonate supported on a silica-propylpiperazine matrix was observed by Oliveira et al. (1998).
The decomposition of H2O2 is an undesirable reaction in the direct H2O2 synthesis process from H2 and O2, while it is a desirable reaction for the generation of hydroxyl radicals in advanced oxidation processes. Thus, it is important to understand how the reaction medium conditions affect the H2O2 decomposition. In other words, to improve the efficiency of the H2O2 production and the advanced oxidation processes are necessary to know the kinetics of H2O2 decomposition and the factors that affect its decomposition. In this work, the H2O2 decomposition was studied on Pd supported on activated carbon, Pd/ZrO2, and Pd/ -Al2O3 between 293 K and 313 K.
-Al2O3 between 293 K and 313 K.
II. METHODS
A. Catalyst Preparation
The supports used in this work were zirconia (ZrO2) supplied by Daiichi Kigenso Kagaku of Japan, alumina ( -Al2O3) from Harshaw, and activated carbon (AC) manufactured by Bonechar, Brazil. The ZrO2 and
-Al2O3) from Harshaw, and activated carbon (AC) manufactured by Bonechar, Brazil. The ZrO2 and  -Al2O3 were calcined in static air at 873 K for 10 h, while AC was washed in distilled water to remove the impurities. The washed AC was then dried at 393 K for 12 h.
-Al2O3 were calcined in static air at 873 K for 10 h, while AC was washed in distilled water to remove the impurities. The washed AC was then dried at 393 K for 12 h.
The 1.0% Pd/ -Al2O3, 1.0% Pd/ZrO2, 5.0% Pd/AC, and 0.5% Pd/AC catalysts were prepared by incipient wetness with aqueous solutions of Pd(NO3)2 (Aldrich Chem. Co.). The Pd(NO3)2.2H2O solution was prepared by dissolving Pd(NO3)2 in concentrated HNO3 (Merck) and distilled water. After impregnation with the aqueous solutions the solids were dried at 393 K for 24 h and calcined at 773 K for 10 h. The catalysts were reduced in flowing H2 (White Martins, 99.999%) at 573 K for 3 h. The amount of Pd in the samples was determined by inductively coupled plasma atomic emission spectroscopy (ICP-AES).
-Al2O3, 1.0% Pd/ZrO2, 5.0% Pd/AC, and 0.5% Pd/AC catalysts were prepared by incipient wetness with aqueous solutions of Pd(NO3)2 (Aldrich Chem. Co.). The Pd(NO3)2.2H2O solution was prepared by dissolving Pd(NO3)2 in concentrated HNO3 (Merck) and distilled water. After impregnation with the aqueous solutions the solids were dried at 393 K for 24 h and calcined at 773 K for 10 h. The catalysts were reduced in flowing H2 (White Martins, 99.999%) at 573 K for 3 h. The amount of Pd in the samples was determined by inductively coupled plasma atomic emission spectroscopy (ICP-AES).
B. Catalyst Characterization
The surface area of the AC, ZrO2 and  -Al2O3 was determined by N2 adsorption at 77 K by the BET method (Brunauer et al., 1938) and the percentage of exposed palladium atoms, FA, was calculated through the titration of preadsorbed oxygen with H2.
-Al2O3 was determined by N2 adsorption at 77 K by the BET method (Brunauer et al., 1938) and the percentage of exposed palladium atoms, FA, was calculated through the titration of preadsorbed oxygen with H2.
Titration of preadsorbed oxygen with H2 was measured in a Micromeritics-ASAP 2010C. The gases used were H2 (White Martins, 99.999%), O2 (White Martins, 99.999%), He (White Martins, 99.9999%), and N2 (White Martins, 99.999%). All gases were used as received. The amount of catalyst used in each experiment varied between 0.50 and 2.00 g. The sample was reduced in flowing H2 (60 cm3min-1) at 573 K for 2 h, followed by evacuation at the same temperature for 2 h. The sample was then cooled to 373 K in vacuo and was further evacuated at this temperature for 0.5 h. Then, O2 was introduced to the sample cell and an adsorption isotherm was recorded between 0.66 and 26.66 kPa. Finally, the sample was evacuated at 373 K for 0.5 h, and H2 was used to titrate the chemisorbed oxygen on Pd. A second adsorption isotherm was recorded after evacuation of the sample at 373 K for 15 minutes. The gas uptake was determined as the intercept of the linear region of the isotherm.
The bulk structure of the samples was determined by the powder X-ray diffraction, XRD, using a Philips model X'Pert diffractometer, with Cu Karadiation, operated at 40 kV and 40 mA.
C. Kinetic Measurements
The rate of H2O2 decomposition was measured using a volumetric method by measuring the volume of O2 evolving from the reaction. All reactions were carried out at atmospheric pressure and over the range of 293 K-323 K. Between 50 and 200 mg of catalyst were dispersed in 250 mL of deionized water, and the reaction system was maintained at the desired temperature in a water bath. The mixture was stirred mechanically with Teflon blades. Then, the H2O2 decomposition was started by injecting between 1 mL and 10 mL of H2O2 into the reaction system. The O2 that evolved during the reaction was measured in different time intervals for at least 1 h.
In order to study the influence of the treatment applied to the Pd catalysts in its H2O2 decomposition activity, the Pd catalyst was treated by H2 and deionized water before the reaction. In addition, successive reactions of H2O2 decomposition were carried out on the same catalyst. For the catalysts treated with H2, the system was purged in flowing pure N2 for at least 1 h and the catalyst was reduced in flowing H2 (2.0 cm3 s-1) at 298 K for 1 h. Then, the H2 was switched to pure N2 and the H2O2 decomposition was started after the system had reached the desired temperature. The catalyst treatment using deionized water was carried out by adding between 50 and 200 mg of catalyst in 500 mL of water maintained under vigorous stirring for 1 h at 298 K. Some catalysts were used for successive reactions. The procedure used in this case was the same procedure applied to the catalysts used in single reactions.
The intra-particle mass transfer effect on the H2O2 decomposition was studied by carrying out the reaction at 313 K using a size of catalyst in the range of 38-208 mm, while the external mass transfer effect was computed using different stirrer speeds.
The reaction temperature for the decomposition of H2O2 on Pd catalysts was varied between 293 and 323 K, and the initial concentration of H2O2 was fixed at 0.062 mol/L for the calculation of the apparent activation energy. To determine the reaction order with respect to H2O2, the initial concentration of H2O2 was varied between 0.050 and 0.300 mol/L.
III. RESULTS AND DISCUSSION
The specific surface areas of the AC, ZrO2 and  -Al2O-3 were about 550, 20, and 160 m2g-1, respectively. The amount of Pd determined by ICP-AES was 0.98%, 0.95%, 5.1%, and 0.49% for the 1.0% Pd/
-Al2O-3 were about 550, 20, and 160 m2g-1, respectively. The amount of Pd determined by ICP-AES was 0.98%, 0.95%, 5.1%, and 0.49% for the 1.0% Pd/ -Al2O3, 1.0% Pd/ZrO2, 5.0% Pd/AC, and 0.5% Pd/AC catalysts, respectively. These results showed a good agreement between the Pd added to the catalyst and that measured by ICP-AES, indicating that the catalyst preparation was conducted well. The FA values as measured by titration of preadsorbed oxygen with H2 were 53.0%, 34.0%, 11.0%, and 58.0% for 1.0% Pd/
-Al2O3, 1.0% Pd/ZrO2, 5.0% Pd/AC, and 0.5% Pd/AC catalysts, respectively. These results showed a good agreement between the Pd added to the catalyst and that measured by ICP-AES, indicating that the catalyst preparation was conducted well. The FA values as measured by titration of preadsorbed oxygen with H2 were 53.0%, 34.0%, 11.0%, and 58.0% for 1.0% Pd/ -Al2O3, 1.0% Pd/ZrO2, 5.0% Pd/AC, and 0.5% Pd/AC catalysts, respectively.
-Al2O3, 1.0% Pd/ZrO2, 5.0% Pd/AC, and 0.5% Pd/AC catalysts, respectively.
The XRD analysis for 0.5% Pd/AC, 1.0% Pd/ -Al2O3, and 1.0% Pd/ZrO2 indicated no presence of metallic Pd, suggesting that the size of the Pd particles are so small (< 5 nm) or amorphous that they cannot be analyzed by x-ray diffraction. In fact, the FA value for 1.0% Pd/
-Al2O3, and 1.0% Pd/ZrO2 indicated no presence of metallic Pd, suggesting that the size of the Pd particles are so small (< 5 nm) or amorphous that they cannot be analyzed by x-ray diffraction. In fact, the FA value for 1.0% Pd/ -Al2O3 suggests a Pd particle size of 2.11 nm, while Pd particle sizes of 3.29 nm and 1.93 nm were calculated for 1.0% Pd/ZrO2 and 0.5% Pd/AC, respectively. For the 5.0% Pd/AC catalyst, a FA value of 11.0% was obtained, which corresponds to a particle size of 10.18 nm. The particle size determined by XRD was 12.0 nm for the 5.0% Pd/AC catalyst, indicating a good agreement between the particle sizes measured by chemisorption and XRD. Thus, the FA values obtained by titration of preadsorbed oxygen with H2 were in good agreement with the results observed in XRD analysis. Metallic Pd was observed by XRD for 5.0% Pd/AC treated with H2, indicating that PdO was reduced to metallic Pd by H2.
-Al2O3 suggests a Pd particle size of 2.11 nm, while Pd particle sizes of 3.29 nm and 1.93 nm were calculated for 1.0% Pd/ZrO2 and 0.5% Pd/AC, respectively. For the 5.0% Pd/AC catalyst, a FA value of 11.0% was obtained, which corresponds to a particle size of 10.18 nm. The particle size determined by XRD was 12.0 nm for the 5.0% Pd/AC catalyst, indicating a good agreement between the particle sizes measured by chemisorption and XRD. Thus, the FA values obtained by titration of preadsorbed oxygen with H2 were in good agreement with the results observed in XRD analysis. Metallic Pd was observed by XRD for 5.0% Pd/AC treated with H2, indicating that PdO was reduced to metallic Pd by H2.
In order to verify the external mass transfer limitations, the H2O2 decomposition was carried out at 313 K using different stirrer speeds. We observed that the reaction rate was not affected by the stirrer speeds, indicating that the H2O2 decomposition was not limited by external mass transfer. The intra-particle mass transfer limitation was observed in this work for a particle size larger than 125 mm. Thus, the experiments were carried out with a particle size between 38 and 125 mm to avoid mass transfer effects on the reaction rate.
Figure 1 shows the variation of H2O2 concentration as a function of reaction time for an initial H2O2 concentration of 0.062 mol/L. (H2O2)0 denotes the initial H2O2 concentration and (H2O2) is the H2O2 concentration at time t. The H2O2 concentration decreased with the increasing reaction time. The H2O2 concentration decreased from 0.062 to 0.018 in 180 minutes for the 5.0% Pd/AC catalyst, while a decrease of 0.062 to 0.005 in 80 minutes was observed for the 0.5% Pd/AC catalyst. For 1.0% Pd/ -Al2O3 and 1.0% Pd/ZrO2 catalysts, the decrease in H2O2 concentration as a function of time was very similar. We observed a decrease in the H2O2 concentration of 86% and 79% in 90 minutes of reaction for the 1.0% Pd/
-Al2O3 and 1.0% Pd/ZrO2 catalysts, the decrease in H2O2 concentration as a function of time was very similar. We observed a decrease in the H2O2 concentration of 86% and 79% in 90 minutes of reaction for the 1.0% Pd/ -Al2O3 and 1.0% Pd/ZrO2 catalysts, respectively. The decrease in the H2O2 concentration over time was higher for catalysts with higher FA values, indicating that the H2O2 decomposition depended on the surface area of Pd. Experiments with initial H2O2 concentration in the range of 0.048-0.300 mol/L showed similar behavior that one using initial H2O2 concentration of 0.062 mol/L. A plot of Ln[(H2O2)0/(H2O2)] as a function of time was made for all catalysts using initial H2O2 concentration of 0.062 mol/L (Fig. 2). The results showed a straight line for all catalysts, indicating a first-order kinetic law in relation to H2O2 concentration. Thus, the reaction rate increased linearly when the initial H2O2 concentration was increased.
-Al2O3 and 1.0% Pd/ZrO2 catalysts, respectively. The decrease in the H2O2 concentration over time was higher for catalysts with higher FA values, indicating that the H2O2 decomposition depended on the surface area of Pd. Experiments with initial H2O2 concentration in the range of 0.048-0.300 mol/L showed similar behavior that one using initial H2O2 concentration of 0.062 mol/L. A plot of Ln[(H2O2)0/(H2O2)] as a function of time was made for all catalysts using initial H2O2 concentration of 0.062 mol/L (Fig. 2). The results showed a straight line for all catalysts, indicating a first-order kinetic law in relation to H2O2 concentration. Thus, the reaction rate increased linearly when the initial H2O2 concentration was increased.

Figure 1. Decomposition of H2O2 on supported Pd catalysts at 303 K.

Figure 2. Decomposition kinetics of H2O2 on supported Pd catalysts at 303 K.
In order to verify the influence of the catalyst treatment using deionized water on H2O2 decomposition, the 5.0% Pd/AC catalyst was used in this reaction at 293 K and 303 K. The initial concentration of H2O2 decreased as the reaction time increased in both temperatures, as shown in Fig. 3. However, the decrease in the initial concentration of H2O2 was faster for the catalysts treated with deionized water, indicating that this treatment can remove adsorbed species on the surface of the catalyst, thus increasing the reaction rate. Similar behavior was observed for the Pd catalysts treated with H2 (Fig. 4) and we observed that the decrease in the concentration of H2O2 was much greater for the catalysts treated with H2 than for the untreated catalysts. The treatment of oxidized Pd catalysts with H2 leads to the formation of metallic Pd, as observed by XRD. In fact, PdO reduction by H2 has been studied by several authors (Crozier et al., 1998; Okumura and Niwa, 2000; Yang et al., 2002; Takeguchi et al., 2003), who observed that the PdO can be reduced to metallic Pd at room temperature. Thus, the high H2O2 decomposition rate on Pd catalyst treated with H2 can be attributed to the presence of metallic Pd, which is more active in H2O2 decomposition. Similar results were found by other authors (Choudhary et al., 2001; 2002; 2006) in the H2O2 decomposition on supported Pd catalysts.

Figure 3. Effect of the catalyst treatment with distilled water on the decomposition of H2O2 on 5.0% Pd/AC.

Figure 4. Effect of H2 treatment on the activity of Pd/AC catalysts for the decomposition of H2O2 at 293 K.
Successive reactions of H2O2 decomposition on 5.0% Pd/AC and 0.5% Pd/AC catalysts were carried out without catalyst exchange at 298 K, and the results are shown in Fig. 5. We can observe that the H2O2 decomposition activity remained practically constant during four successive reactions of H2O2 decomposition on 5.0% Pd/AC catalyst (Fig. 5A), indicating that the H2O2 decomposition itself causes a small deactivation process in the 5.0% Pd/AC catalyst. For the 0.5% Pd/AC catalyst, the H2O2 decomposition activity decreased slowly after its use in each successive reaction, suggesting a deactivation of the catalyst (Fig. 5B). The deactivation of the 0.5% Pd/AC and 5.0% Pd/AC catalysts can be attributed to the oxidation of Pd by H2O2. This deactivation can suggest a different oxidation rate of Pd by H2O2, and this oxidation appears to depend on the particle size of the catalyst. Other works (Lieske and Volter, 1985; Tobias et al., 2006) have shown that the oxidation of Pd by O2 depends on the particle size of Pd, and small particles are easily oxidized. Thus, the deactivation of the catalyst in the successive H2O-2 decompositions appear to depend on the particle size of the catalyst, and small particles tend to have a high deactivation process, as observed in Fig. 5. A similar behavior was found in both the 1.0% Pd/Al2O3 and 1.0% Pd/ZrO2 catalysts.

Figure 5. Effect of successive reactions on the decomposition of H2O2 at 303 K on (A) 5.0% Pd/AC and (B) 0.5% Pd/AC.
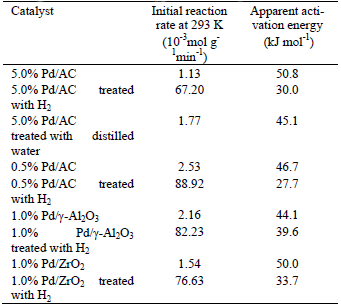
In all the experiments performed in this work, we observed that the reaction rate of H2O2 decomposition on supported Pd catalysts can be described by a first-order kinetic law in relation to H2O2 concentration (Fig. 2). Table 1 shows the reaction rate measured at 293 K using an initial concentration of H2O2 of 0.062 mol/L and the apparent activation energy calculated for H2O2 decomposition on supported Pd catalysts. The reaction rate for the catalysts treated with H2 varied between 67×10-3 and 90×10-3 mol g-1min-1, while for the Pd catalysts that were not treated, the reaction rate varied between 1.10×10-3 and 2.60×10-3 mol g-1min-1. The Pd catalysts treated with H2 showed a reaction rate 35-50 fold higher than the Pd catalysts not treated with H2 (Table 1). This fact can be attributed to the presence of metallic Pd due to the reduction of PdO to Pd by H2. Other authors (Choudhary et al., 2001; 2002; 2006) have observed similar results. The apparent activation energy varied between 30 and 50 kJmol-1 for Pd catalysts. These values are high when compared to the 17.65 kJmol-1 obtained by Choudhary and Gaikwad (2003). However, Voloshin et al. (2008) found a value of 70.9 kJmol-1 for the apparent activation energy for the decomposition of H2O2 on Pd/SiO2. Ariafard et al. (2003) obtained apparent activation energy values between 32 and 41 kJmol-1 for H2O2 decomposition on La0.9Sr0.1Ni1-×Cr×O3 perovskites. Apparent activation energy values between 30 and 60 kJ/mol have also been found in other works (Lin and Gurol, 1998; Oliveira et al., 1998). Thus, the apparent activation energy values found in this work are in good agreement with the values found in other works. All Pd catalysts showed high catalytic activity for the H2O2 decomposition. However, the H2O2 decomposition activity was higher for the 0.5% Pd/AC treated with H2. Thus, the activity of the catalytic decomposition of H2O2 depends on the treatment applied to the catalyst.
Table 1. Reaction rate and apparent activation energy for the decomposition of H2O2 on supported Pd catalysts.
IV. CONCLUSIONS
In this work, the influence of process parameters on H2O2 decomposition were studied for the supported Pd catalysts. The H2O2 decomposition depends on the treatment applied to the catalysts. The catalysts treated with H2 resulted in a larger rate of H2O2 decomposition, indicating a reduction of PdO to metallic Pd by H2. This reduction was confirmed by XRD. The treatment with distilled water had a small effect on the rate of H2O2 decomposition, suggesting a removal of impurities on the catalyst surface. After four successive decompositions of H2O2, we observed a small deactivation for the Pd/AC catalysts. This fact was attributed to oxidation of metallic Pd to PdO by H2O2.
All reactions were well represented by a first-order rate law with respect to H2O2. The apparent activation energy varied between 27 and 40 kJ/mol for the supported Pd catalysts treated with H2, while for the supported Pd catalysts not treated with H2, the apparent activation energy values were close to 50 kJ/mol. The H2O2 decomposition activity was higher for the 0.5% Pd/AC treated with H2, indicating that the metallic Pd is more active than the PdO for the H2O2 decomposition.
REFERENCES
1. Ariafard, A., H.R.Aghabozorg and F.Salehirad, "Hydrogen peroxide decomposition over La0.9Sr0.1Ni1-WCrWO3 perovskites,"Catal. Commun., 4, 561-566 (2003). [ Links ]
2. Brunauer, S., P.H. Emmett and E. Teller, "Adsorption of Gases in Multimolecular Layers," J. Am. Chem. Soc., 60, 309-319 (1938). [ Links ]
3. Chou, S. S. and C.P. Huang, "Decomposition of hydrogen peroxide in a catalytic fluidized-bed reactor," Appl. Catal.A:Gen., 185, 237-245 (1999). [ Links ]
4. Choudhary, V. R. and A.G. Gaikwad, "Kinetics of hydrogen peroxide decomposition in aqueous sulfuric acid over palladium/carbon: Effect of acid concentration," React. Kinet. Catal. Lett. 80, 27-32 (2003). [ Links ]
5. Choudhary, V. R., A.G. Gaikwad and S.D. Sansare, "Nonhazardous direct oxidation of hydrogen to hydrogen peroxide using a novel membrane catalyst," Angew. Chem. Int. Ed., 40, 1776-1779 (2001). [ Links ]
6. Choudhary, V.R., A.G. Gaikwad and S.D. Sansare, "Activation of supported Pd metal catalysts for selective oxidation of hydrogen to hydrogen peroxide," Catal. Lett. 83, 235-239 (2002). [ Links ]
7. Choudhary, V.R. and P. Jana, "Direct oxidation of H2 to H2O2 over PdO/Al2O3 catalysts in aqueous acidic medium: Influence on H2O2 formation of Pd loading, calcination temperature and reduction of catalyst and presence of halide anions," Catal. Commun. 9, 2371-2375 (2008). [ Links ]
8. Choudhary, V.R. and P. Jana, "Direct H-2-to-H2O2 oxidation over highly active/selective Br-F-Pd/Al2O3 catalyst in aqueous acidic medium: Influence of process conditions on the H2O2 formation," Appl. Catal.A:Gen., 352, 35-42 (2009). [ Links ]
9. Choudhary, V.R., C. Samanta, and T.V. Choudhary, "Direct oxidation of H2 to H2O2 over Pd-based catalysts: Influence of oxidation state, support and metal additives," Appl. Catal.A:Gen., 308, 128-133 (2006). [ Links ]
10. Choudhary, V. R., C. Samanta and P. Jana, "Decomposition and/or hydrogenation of hydrogen peroxide over Pd/Al2O3 catalyst in aqueous medium: Factors affecting the rate of H2O2 destruction in presence of hydrogen," Appl. Catal.A:Gen., 332, 70-78 (2007). [ Links ]
11. Crozier, P.A., R. Sharma and A.K. Datye, "Oxidation and reduction of small palladium particles on silica," Microsc. Microanal., 4, 278-285 (1998). [ Links ]
12. Dantas, T.L.P., V.P. Mendonca, H.J. Jose, A.E. Rodrigues and R. Moreira, "Treatment of textile wastewater by heterogeneous Fenton process using a new composite Fe2O3/carbon," Chem. Eng. J., 118, 77-82 (2006). [ Links ]
13. Gurol, M.D. and S.S. Lin, "Hydrogen peroxide/iron oxide-induced catalytic oxidation of organic compounds," J. Adv. Oxid. Technol., 5, 147-154 (2002). [ Links ]
14. Hermanek, M., R. Zboril, N. Medrik, J. Pechousek and C. Gregor, "Catalytic efficiency of iron(III) oxides in decomposition of hydrogen peroxide: Competition between the surface area and crystallinity of nanoparticles," J. Am. Chem. Soc., 129, 10929-10936 (2007). [ Links ]
15. Huang, H. H., M.C. Lu and J.N. Chen, "Catalytic decomposition of hydrogen peroxide and 2-chlorophenol with iron oxides," Water Res., 35, 2291-2299 (2001). [ Links ]
16. Landon, P., P.J. Collier, A.F. Carley, D. Chadwick, A.J. Papworth, A. Burrows, C.J. Kiely and G.J. Hutchings, "Direct synthesis of hydrogen peroxide from H2 and O2 using Pd and Au catalysts, Phys. Chem. Chem. Phys., 5, 1917-1923 (2003). [ Links ]
17. Lieske, H. and J. Volter, "Pd redispersion by spreading of PdO in O2 treated Pd/Al2O3," J. Phys. Chem., 89, 1841-1842 (1985). [ Links ]
18. Lin, S. S. and M.D. Gurol, "Catalytic decomposition of hydrogen peroxide on iron oxide: Kinetics, mechanism, and implications," Environ. Sci. Technol., 32, 1417-1423 (1998). [ Links ]
19. Lopez, F., M.J. Diaz, M.E. Eugenio, J. Ariza, A. Rodriguez and L. Jimenez, "Optimization of hydrogen peroxide in totally chlorine free bleaching of cellulose pulp from olive tree residues," Bioresour. Technol., 87, 255-261 (2003). [ Links ]
20. Okumura, K. and M. Niwa, "Regulation of the Dispersion of PdO through the Interaction with Acid Sites of Zeolite Studied by Extended X-ray Absorption Fine Structure," J. Phys. Chem. B, 104, 9670-9675 (2000). [ Links ]
21. Oliveira, S. F., J.G.P. Espinola, W.E.S. Lemus, A.G. de Souza and C. Airoldi, "Kinetic study of the decomposition of hydrogen peroxide catalysed by Co(II) acetylacetonate supported on a silica-propylpiperazine matrix," Colloids Surf. A:Physicochem. Eng. Asp., 136, 151-154 (1998). [ Links ]
22. Perathoner, S. and G. Centi, "Wet hydrogen peroxide catalytic oxidation (WHPCO) of organic waste in agro-food and industrial streams," Top. Catal., 33, 207-224 (2005). [ Links ]
23. Petlicki, J., D. Palusova and T.G.M. van de Ven, "Physicochemical aspects of catalytic decomposition of hydrogen peroxide by manganese compounds," Ind. Eng. Chem. Res. 44, 2002-2010 (2005). [ Links ]
24. Pignatello, J. J., "Dark and photoassisted Fe3+ catalyzed degradation of chlorophenoxy herbicides by hydrogen peroxide," Environ. Sci. Technol., 26, 944-951 (1992). [ Links ]
25. Shivankar, V. S. and N.V. Thakkar, "Decomposition of hydrogen peroxide in presence of mixed ligand cobalt(II) and nickel(II) complexes as catalysts," J. Sci. Ind. Res., 64, 496-503 (2005). [ Links ]
26. Simmons, G. F., J.L. Smilanick, S. John and D.A. Margosan, "Reduction of microbial populations on prunes by vapor-phase hydrogen peroxide," J. Food Prot., 60, 188-191 (1997). [ Links ]
27. Sisco, J. C., B.L. Austin, J.S. Mok and W.E. Anderson, "Autoignition of kerosene by decomposed hydrogen peroxide in a dump-combustor configuration," J. Propul. Power, 21, 450-459 (2005). [ Links ]
28. Takeguchi, T., O. Takeoh, S. Aoyama, J. Ueda, R. Kikuchi and K. Eguchi, "Strong chemical interaction between PdO and SnO2 and the influence on catalytic combustion of methane," Appl. Catal.A: Gen., 252, 205-214 (2003). [ Links ]
29. Tobias, S., B.Bjorn, E.S.David, L.Mathias, K.S.Shamil, S.Swetlana, L.Jorg and F. Hans-Joachim, "Size-Dependent Oxidation Mechanism of Supported Pd Nanoparticles13,"Angew. Chem. Int. E., 45, 3693-3697(2006). [ Links ]
30. Verce, M. F., B. Jayaraman, T.D. Ford, S.E. Fisher, A.J. Gadgil and T.M. Carlsen, "Minimizing decomposition of vaporized hydrogen peroxide for biological decontamination of galvanized steel ducting," Environ. Sci. Technol., 42, 5765-5771 (2008). [ Links ]
31. Voloshin, Y., J. Manganaro and A. Lawal, "Kinetics and Mechanism of Decomposition of Hydrogen Peroxide over Pd/SiO2 Catalyst," Ind. Eng. Chem. Res., 47, 8119-8125 (2008). [ Links ]
32. Wojciak, A., H. Kasprzyk, I. Khmelinskii, A. Krawczyk, A.S. Oliveira, L.F.V. Ferreira, A. Weselucha-Birczynska and M. Sikorski, "Direct characterization of hydrogen peroxide bleached thermomechanical pulp using spectroscopic methods," J. Phys. Chem. A, 111, 10530-10536 (2007). [ Links ]
33. Yang, L., C. Shi, X. He and J. Cai, "Catalytic combustion of methane over PdO supported on Mg-modified alumina," Appl. Catal. B: Environ., 38, 117-125 (2002). [ Links ]
34. Zazo, J. A., J.A. Casas, A.F. Mohedano and J.J. Rodriguez, "Catalytic wet peroxide oxidation of phenol with a Fe/active carbon catalyst," Appl. Catal. B:Environ., 65, 261-268 (2006). [ Links ]
Received: June 7, 2010
Accepted: November 3, 2010
Recommended by subject editor: Orlando Alfano













