Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Latin American applied research
versión impresa ISSN 0327-0793versión On-line ISSN 1851-8796
Lat. Am. appl. res. vol.42 no.1 Bahía Blanca ene. 2012
Activation of HMDSO thin films with low pressure argon plasma and vacuum ultraviolet radiation
T. García-Pérez†, E. Rodríguez‡, E. Bittencourt† and Z. Jova†
†Faculty of Chemical Eng., Department of Polymers Technology, UNICAMP, Campinas, SP, Brazil.
tsaigarcia@feq.unicamp.br
‡Institute of Physics, Department for Quantum Electronics, UNICAMP, Campinas, SP, Brazil.
Abstract— Polymeric surfaces obtained by chemical vapor deposition of HMDSO on aluminum plates using plasma were modified by means of low pressure Argon plasma and vacuum ultraviolet radiation (VUV). The polymeric surface was directly exposed to the plasma -which also acted as a vacuum ultraviolet radiation source- at different conditions. An important contribution of the VUV radiation component in surface modification was observed. The surface free-energy, the chemical composition, and the morphology of the films were determined by contact angle measurement, FTIR spectroscopy, and SEM. It was observed that the action of VUV radiation inside de Argon plasma was responsible for more than 45% of the increase of surface free-energy, and that the influence of VUV radiation on surface free-energy varied significantly as consequence of different exposure times.
Keywords— Hexamethyldisiloxane Films; Vacuum Plasma; Vacuum Ultraviolet Radiation; Surface Modification.
I. INTRODUCTION
Due to their chemical nature, polymeric materials frequently exhibit poor adhesive properties (Miller et al., 2000; Shenton and Stevens, 2001; Werthermeir et al., 1999). However, adhesive properties are a critical factor in industrial applications where these materials require high physical bonding either with other polymers or with metals. The control of adhesive properties of polymeric surfaces is importance in industries such as automotive, aeronautical, microelectronics, and packaging.
The improvement of adhesion properties of polymeric surfaces is frequently achieved by means of pre-treatments that eliminate weak boundary layers and modify the chemical composition of surfaces. In many cases, these treatments utilize mechanical (e.g. sand-blasting) or high pollutant wet-chemical processes (Deshmukh and Bhat, 2003; Yi et al., 2003). The quality of the surfaces resulting from these methods is in many cases difficult to control.
Several studies describing the use of plasma to activate the surface of different materials as a pre-treatment to improve adhesion have been reported (Terlingen, 2004; Awaja et al., 2009). However, it is not well known the effect of the VUV radiation component present in Argon plasma.
Plasma treatment process is a clean and efficient alternative to replace traditional pretreatment processes of materials (D´Agostino, 1993; Bertrand et al., 1998, Han et al., 2007). It uses few reagents and eliminates the necessity of water, hence reducing pollution significantly. It, additionally, is versatile, allowing a variety of processes, such as surface cleaning, deposition of thin polymer films, and surface functionalization, in single equipment.
Siloxanesnare common industrial adhesion promoters (Salamone, 1996; Petrie, 2000). Hexamethyldisiloxane (HMDSO) promotes anti-corrosive behavior in a material when a HMDSO film is deposited on the surface of metals by means of plasma (Vautrin et al., 2000; Fernandes et al., 2002). This siloxane presents additional advantages for industrial applications: low toxicity, easy manipulation, and commercial availability. Nevertheless, HMDSO presents a hydrophobic surface that interferes the adhesion process.
This paper's aim is to describe a process to modify HMDSO surfaces and to increase HMDSO's surface free-energy, as a way to promote HMDSO adhesion properties. The process consisted on the deposition of HMDSO films on aluminum plates cleaned by plasma, followed by the modification of its superficial and chemical properties using plasma and VUV radiation.
II. EXPERIMENTAL
The surfaces of aluminum plates were cleaned with Argon plasma. The HMDSO film was deposited in a second step, followed by the activation of HMDSO film surface by low pressure Argon plasma and ultraviolet (VUV) radiation. The surface properties were characterized before and after the activation process by contact angle, Fourier Transform Infrared Spectroscopy (FTIR), and Scanning Electron Microscopy (SEM).
A. Production of HMDSO films.
The production of the films was performed by means of the plasma enhanced chemical vapor deposition (PECVD) technique, using HMDSO as precursor gas and Argon (99.999%) as carrier gas. Aluminum plates (Al5000 series alloy, 30x60x1 mm) were used as substrate. The temperature of the substrate during films growth was 300 K.
The plasma equipment utilized was designed and constructed at the Institute of Physics Gleb Watanghin, State University of Campinas (UNICAMP) (Rodriguez, 2004). Figure 1 presents a scheme of the installation.

Fig. 1. Schematic of the plasma equipment used to deposit the HMDSO films.
The Radio Frequency (RF) field was generated between two electrodes with cooper positive electrode. The source TOKYO HI-Power RF-150, connected to the matching box, supplies a RF signal at 13.56 MHz.
The two RF electrodes concentrate the plasma in the central region of the chamber. The HMDSO flow was controlled using a fine control needle valve (Edwards LV10K).
The surface of the aluminum substrate was cleaned, in a first step, using Argon plasma. Cleaning work started at 7.5×10-5 Torr and it was followed by the introduction of the cleaning agent, Argon, until the pressure increased up to 0.13 Torr. The power supplied was 40 W and the cleaning time was 5 minutes.
The work of D´Agostino (1993) served as primary reference to choose the range of parameters utilized for the deposition of HMDSO films. The parameters reported by this author were: pressure of HMDSO 0.03 Torr, power 30 W, and frequency of 13.56 MHz. Several preliminary experiments were carried out to determine the appropriate growth process parameters for the equipment. The following ranges were explored: HMDSO pressure between 0.02 and 0.03 Torr; Argon pressure from 0.15 Torr; Power from 30 to 60W; deposition time from 3 to 30 minutes. FTIR (Transmittance) tests of plates served to identify the best working conditions. These conditions were: HMDSO Pressure of 0.03 Torr, Argon Pressure of 0.15 Torr, Power of 30 W, and deposition time of 15 minutes. These deposition parameters were used in all the tests.
B. Modification of deposited films.
The process of activation of HMDSO surface was carried out using two techniques: low pressure plasma, and VUV radiation.
Treatment by low pressure Argon plasma.
The aluminum plates coated with the HMDSO layer were placed into the activation chamber. The vacuum pumps were turned on at a pressure of 7.5×10-5 Torr, followed by the feeding of Argon until 0.15 Torr of pressure. Treatment times between 15 and 60 seconds and power between 30 and 60 W were used. The RF source was operated with a selected group of parameters resulting from an experimental design (pressure, time, and power). The plasma was directly applied to the HMDSO layer.
Treatment with VUV radiation using the Argon plasma as VUV source
Low pressure Argon plasma was used as source of VUV radiation (100 - 200 nm). A VUV radiation filter made of Lithium Fluoride (LiF) (radius = 16.4 mm and 4 mm thickness) was used to isolate the influence of the ultraviolet radiation within Argon plasma. The LiF crystal has excellent transmittance in the VUV range between 0.104 and 7μ (Del Mar Photonics, 2006) acting as a barrier to avoid the influence of ions, meta-stable species, and radicals present in the plasma. The LiF filter was placed over a Teflon ring designed to maintain the filter 1 mm over the sample surface, which helped to avoid the contact filter-metal surface. This additionally permitted to generate the vacuum in the gap filter - sample.
As shown in Fig. 2, the LiF crystal covered only a part of the sample surface. This allowed us to compare the surface's properties in the region completely exposed to the action of the plasma with the surface exposed to the action of VUV radiation.

Fig. 2. LiF crystal position on HMDSO film.
C. Characterization of the deposited and modified films
Thickness, optical absorption and contact angle of deposited films were determined. The chemical composition and morphology were studied by FTIR, and by SEM. For the modified films, the characterization included contact angle, FTIR and SEM.
The thickness and refractive index measurements of deposited films were carried out using a prism coupling system model METRICON 2010. The deposition rate was calculated as the ratio of film thickness to deposition time. The film absorption spectrum was measured using a spectrophotometer (Perkin Elmer- Lamda 9).
Contact angle
According to Fadda and Clarisse (1995), the measurement of the contact angle is sensible to superficial modifications in the range of 20 to 30 Å of depth. The contact angles of resulting surfaces were measured before and after each treatment and were used to calculate the surface free-energy. The results permitted to determine the influence of each treatment in the surface of HMDSO coatings. Surface free energy was determined by the method of Owens and Wendt (1969) using solutions of de-ionized water and ethylene glycol.
FTIR measurements
The FTIR transmittance measurements were performed from 400 to 4000 cm-1, using a Digilab Serie Scimitar spectrometer. Measurements were conducted by reflectance, using a 10 SPEC (10 Degree Specular Reflactance) accessory.
SEM measurements.
Morphological analyses were carried out by SEM using a Leo 440i electron microscope with backward-scattering detector. The aluminum plates were analyzed in the presence and absence of deposited film, as well as, before and after activation.
III. RESULTSHMDSO films of 943 ±5 nm thickness were obtained after 15 minutes of deposition (the deposition rate was 62.8 nm/min). The refractive index (for λ=633 nm) was n=1.633 ±0.001.
Figure 3 shows the typical absorption spectra of HDMSO films. The optical absorption spectrum shown in Fig. 4 confirms that the films absorbed radiation with wavelength lower than 330 nm, so the film surface can be modified under the action of radiation in this range of wavelength.

Fig. 3. Optical absorption spectrum of HMDSO films. (Deposition parameters: 13.56MHz, 30 W, Argon: 0.15 Torr, HMDSO: 0.03 Torr.)

Fig. 4. Surface free-energy after Argon plasma treatment and after VUV treatment for different times of exposition.
A. HMDSO films surface free energy.
Contact angles were measured before and after treatments to investigate the influence of low pressure Argon plasma and VUV treatment on surface free-energy. The results are presented in Table 1. It is observed that the contact angles of de-ionized water decrease as surface free-energy increases after the Argon plasma treatment.
Table 1. Contact angle and surface free-energy in HMDSO films, before and after Argon plasma and VUV treatments.
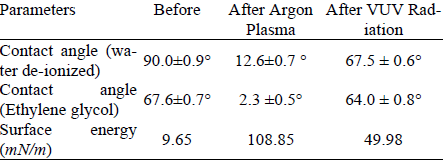
Note: Contact angle results are the average of five measurements.
This behavior was also observed, but in lower degree, under the action of VUV radiation alone. In both cases, the best results occurred at 30 seconds and 60 W. The action of VUV radiation inside the Argon plasma is responsible for up to 45.9% of the effect. The influence of VUV radiation on surface free-energy varies significantly among experiments, as shown in Fig. 4, as consequence of different exposition times.
Both, plasma and VUV treatments favored the reduction of the contact angle in the HMDSO surface and increased the surface free-energy. A reduction in the contact angle means an increase of the humectation of the surface, which enhances further adhesive processes (Dutra, 2002; Awaja et al., 2009).
B. Surface chemical composition analyzed by FTIR
HMDSO films
The FTIR spectrum of a freshly deposited HMDSO film is shown in Fig. 5.

Fig. 5. FTIR spectrum for the HMDSO film polymerized by plasma at: 13.56 MHz, 30W, Argon pressure 0.15 Torr, HMDSO pressure 0.03 Torr.
The FTIR spectrum of resulting films shows an intense broad band corresponding to CH3 and CH2 groups, between 750 and 900 cm-1, and other bands between 1000 and 1150 cm-1 corresponding toSi-O-Si bonds. According to Vautrin et al. (2000), the absorption band of medium intensity observed at 1260 cm-1 can be attributed to the Si-CH3 or Si-CH3 symmetrical bending in Si-(CH3)x. The absorption band of medium intensity observed at 2145 cm-1 wavelength corresponds to the Si-H stretching vibration, normally observed in the band 2100-2250 cm-1 (Vautrin et al., 2000 ). This band results from the molecular fragmentation of Si-CH3 breaking to detach the -CH3 group and then combining with the hydrogen formed from the breaking of C-H bonds (Fang et al., 2001). The band observed between 2874 and 2901 cm-1 can be attributed to the C-H stretching vibrations in methyl groups (Skoog et al., 2002).
HMDSO films modified with Argon treatment
The film modified using Argon plasma was analyzed one hour and one week after treatment (See Fig. 6a and 6b). In the first case, there were no major changes in the shape of FTIR spectra; however, the relative transmittance between different bands was modified. The reduction in transmittance and consequently the increase in opacity show that the sample is more cross-linked than the original.

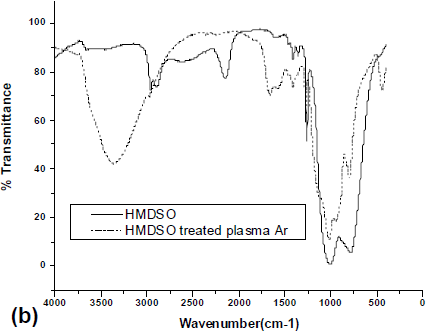
Fig. 6. a) FTIR spectrum for the activated film using low pressure Argon plasma one hour after the treatment b) FTIR spectrum for the activated film using low pressure Argon plasma one week after the Argon plasma treatment.
The treated plates analyzed one week after the treatment (the samples were storage at ambient conditions, 300 K and 98.6 kPa in contact with air) allowed to understand of the nature of surface modification during storage. Results of FTIR presented in Fig. 6b indicate important changes on the surface film, when compared with the original film's surface. A new peak of weak intensity at 436 cm-1 can be attributed to the presence of SiOx (Li et al., 2004). A medium band in the range of 1500-1650 cm-1 can be assigned to C-H angular deformation (Skoog, 2002). A new broad band of medium intensity at 3367 cm-1 corresponding to the -OH functional groups is also observed. Polymer's surface treatment using Argon plasma promotes the creation of free radicals. These radicals could react with oxygen and moisture of air, "which explains the presence of _OH groups in the IR spectrum after Argon plasma treatment" (Strobel et al., 1994). The hydroxyls in the film surface results from the reaction between Si-H groups (identified in the IR spectra for a band at 2145 cm-1) with oxygen of air. Another difference observed between the materials recently treated and the aged ones is the presence of a weak absorption band with maximum at 436 cm-1, corresponding to SiOx groups that are very hydrophilic. These results suggest that the treatment modifies the film hydrophility, which also explains the presence of -OH bands in the IR spectrum of the aged film. The alteration of the band corresponding to the Si-O-Si group occurs between 1000 and 1150 cm-1, indicating the occurrence of alterations in this group after storage.
Other effect of a polymer treatment with plasma is the increase of surface roughness and surface cracking (Vasilets et al., 1998). This process could favor the incorporation of functionalities in the surface, because the increase of roughness increases the surface area in which reactions to incorporate functionalities -OH occur. Thus, the results suggest that low pressure Argon plasma promotes the incorporation of -OH functionalities in reactions occurring in contact with humid air during storage.
HMDSO film with VUV treatment
FTIR results for the films obtained after VUV radiation are presented in Fig. 7. These analyses were carried 1 hour (Fig. 7a) and 1 week after the treatment (Fig. 7b). It can be seen that the VUV radiation did not to produce major chemical modifications on the surface. The chemical composition seems to remain constant one week after the treatment. With the VUV modification, the HMDSO surface does not incorporate water.1

Fig. 7. IR Spectrum for the activated film using VUV radiation into the Argon plasma a) 1 hour after treatment b) 1 week after the VUV treatment.
C. Surface morphology (SEM analyses)
For comparative purposes, SEM analyses were carried out on: a) aluminum substrate, b) the substrate covered with the HMDSO film, c) the HMDSO film treated with Argon plasma, and d) the HMDSO film treated with VUV radiation. The results are presented in Figs. 8, 9, 10 and 11.

Fig. 8. SEM micrographs for aluminum plate without treatment(amplified 5000 times).

Fig. 9. SEM micrographs for Aluminum plate covered with HMDSO film (amplified 5000 times).

Fig. 10. SEM micrographs for HMDSO film treated with low pressure Argon plasma (amplified 5000 times)

Fig. 11. SEM micrographs for HMDSO film treated with VUV radiation (amplified 5000 times).
Figures 8 and 9 show that the deposited film using PECVD covered the aluminum plate, and deposition generates 3D spherical accumulations. These accumulations are of two types: the first is on the layer while the second is under the layer, forming part of it, thus contributing to surface roughness increase.
Figure 10 shows the HMDSO surface modified with low pressure Argon plasma. The 3D spheres on the surface have disappeared, indicating that the Argon plasma exerts a surface cleaning effect. Also, a considerable reduction in the amount of 3D spheres under the surface is observed. The 3D spheres observed in the SEM images correspond to HMDSO deposition. VUV can induce photochemical chain scissions at the polymer surface (Fozza et al., 1999), which could etch the weak boundary layer formed during the HMDSO deposition (Vasilets et al., 1998).
The HMDSO surface modified with VUV radiation is presented in the Fig. 11. Comparing Figs. 10 and 11, it is possible to state that VUV radiation alone produces a level of surface cleaning comparable to the one achieved by Argon plasma. Liston et al. (1991) have reported that VUV irradiation has been identified as one of the most important components in plasma-polymer interactions, because the primary reacting driving plasma- polymer interaction results from VUV radiation. This result shows that VUV radiation -as part of Argon plasma- contributes in the modification of the HMDSO deposited film. The elimination of the weak boundary layer is critic for further adhesion processes.
IV. CONCLUSIONS
The results show that a significant modification of HMDSO surfaces can be achieved by using low pressure Argon plasma and VUV radiation. Important differences between the two treatments were observed when compared the modified surfaces. The Argon plasma action does not modify surface chemistry; nevertheless, it catalyzes a process responsible for the incorporation of -OH functionalities during contact with air. This phenomenon is not observed when used VUV radiation. The modified films by VUV radiation are chemically stable during storage.
The influence of the VUV radiation inside of the plasma varies notably among experiments. From the SEM analysis it can be concluded that the VUV plasma contributes to eliminate weak boundary layers, comparable to those achieved by Argon plasma, promoting considerably the modification of HMDSO surface morphology.
Very important reductions of contact angles were observed. For the Argon plasma treatment the water contact angle reduction was 77.6º and for the VUV radiation the water contact angle reduction was 22.5º. The largest reduction occurred when using low pressure Argon plasma.
The reduction of contact angle and the elimination of weak boundary layer on the surface can improve adhesion forces in a bonded joint. Thus, the treatments performed in this study can be used to improve the adhesive properties of HMDSO films deposited by means of plasma.
ACKNOWLEDGMENT
The authors express their gratitude to CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico - Brasil) for the support received.
REFERENCES
1. Awaja, F., M. Gilbert, G. Kelly, B. Fox and P. Pigram, "Adhesion of polymers," Progress in polymer science, 34, 948-968 (2009). [ Links ]
2. N. Bertrand, B. Drévillon, A. Gheorghiu, C. Sénémaud, L. Martinu and J.E. Klemberg-Sapieha, "Adhesion improvement of plasma-deposited silica thin films on stainless steel substrate studied by x-ray photoemission spectroscopy and in situ infrared elipsometry," J. Vac. Sci. Technology. A16, 116-123 (1998). [ Links ]
3. D'Agostino, R., Plasma deposition, treatment, and etching of polymers. San Diego. Academic Press (1993). [ Links ]
4. Del Mar Photonics, Available online at: http://www.dmphotonics.com/Photonics_Glossary/glossary_navigator.htm, Access on April 20 (2006). [ Links ]
5. Deshmukh, R.R. and N.V. Bhat, "The mechanism of adhesion and printability of plasma processed PET films," Mater. Res. Innov., 7, 283-290 (2003). [ Links ]
6. Dutra, J.C.N., Modificação da Superfície de Borracha EPDM por meio de Processos a Plasma Frio, Doctor Thesis, Faculdade de Engenharia Química, Universidade Estadual de Campinas, Campinas(2002). [ Links ]
7. Fadda, E. and C. Clarisse, "Characterization of the surface modifications of conducting poly(3-octylthiophene) films by contact angle measurements," Synthetic Metals, 72, 99-103 (1995). [ Links ]
8. Fang, J., H. Chen and X. Xu, "Studies on plasma Polymerization of hexamethyldisiloxane in the presence on different carrier gases," Journal of Applied Polymer Science, 80, 1434-1438 (2001). [ Links ]
9. Fernandes, J.C.S, M.G.S. Ferreira, D.B. Haddaw, A. Goruppa, R. Short and D.G. Dixon, "Plasma-polymerized coatings used as pre-treatment for aluminum alloys." Surface and Coatings Technology, 154, 8-13 (2002). [ Links ]
10. Fozza, A.C., J.E. Klemberg-Sapieha and M.R. Wertheimer, "Vacuum Ultraviolet Irradiation of Polymers," Plasmas and Polymers, 4, 183-206 (1999). [ Links ]
11. Han, M.H., J.P. Jegal, K.W. Park, J.H. Choi, H.K. Baik, J.H. Noh, K.M. Song and Y.S. Lim, "Surface modification for adhesion enhancement of PET-laminated steel using atmospheric pressure plasma," Surface and Coatings Technology, 201, 4948 - 4952 (2007). [ Links ]
12. Li, K., O. Gabriel and J. Meichsner, "Fourier transform infrared spectroscopy study of molecular structure formation in thin films during hexamethyldisiloxane decomposition in low pressure RF discharge," Journal of Physics D: Applied Physics, 37, 588-594 (2004). [ Links ]
13. Liston, E.M., L. Martinu and M.R. Wertheimer, "Plasma surface modification of polymers for improved adhesion: a critical review", J. Adhesion Sci. Technol., 7, 1091-1127, (1993). [ Links ]
14. Miller, A.C., M.T.K. Nowlton and J.C. Berg, "The use of UNIFAC for the estimation of adhesion enhancement between polymers and mineral surfaces treated with silane coupling agents," J. Adhesion Sci. Technol., 14, 1471-1484 (2000). [ Links ]
15. Owens, D.K. and R.C. Wendt, "Estimation of the surface free energy of polymers," J. Appl.Polymer Sci., 13, 1741-1747 (1969). [ Links ]
16. Petrie, E.M., Handbook of Adhesives and Sealants, McGraw-Hill(2000). [ Links ]
17. Rodriguez, E., Fabricação de multicamadas de Quantum Dots de PbTe por laser ablation. PhD. Dissertation, Instituto de Física Gleb Wataghin. Universidade Estadual de Campinas (2004). [ Links ]
18. Salamone, J.C., Polymeric materials Encyclopedia, Bausch & Lomb, Rochester, New York, USA, 10, (1996). [ Links ]
19. Skoog, D.A., F.J. Holler and T.A. Nieman, Princípios de análise instrumental, 5ed., Bookman, Porto Alegre (2002). [ Links ]
20. Shenton, M.J. and G.C. Stevens, "Surface modification of polymer surfaces: atmospheric plasma versus vacuum plasma treatmentes," J. Phys. D: Appl.Phys., 34, 2761-2768 (2001). [ Links ]
21. Shin D. H. et al., "Modification of metal surfaces by microwave plasma at atmospheric pressure, Surface and Coatings Technology, 201, 4939-4942 (2007). [ Links ]
22. Strobel, M., C.S. Lyons and K.L. Mittal, Plasma Surface Modification: Relevance to Adhesion.Utrecht, The Netherlands (1994). [ Links ]
23. Terlingen, J.A.G., "Functionalization of surfaces," Europlasma Technical paper, Available online at: http://acms.lodestar.be/europlasma/bestanden/EP%20TP%20Functionalization%20of%20polymer%20surfaces.doc.pdf (2004). [ Links ]
24. Vasilets, V.N., K. Nakamura, Y. Uyama, S. Ogata and Y. Ikada, "Improvement of the micro-wear resistance of silicone by vacuum ultraviolet irradiation," Polymer, 39, 2875-2881 (1998). [ Links ]
25. Vautrin, U., C. Boisse-Laporte and N. Benissad, "Plasma-polymerized coatings using HMDSO precursor for iron protection," Progress in Organic Coatings, 38, 9-15 (2000). [ Links ]
26. Werthermeir, M.R., A.C. Fozza and A. Holländer, "Industrial processing of polymers by low-pressure plasmas: the role of VUV radiation," Nuclear Instruments and Methods in Physics Research Section B: Beam Interaction with Materials and Atoms, 151, 65-75 (1999). [ Links ]
27. Yi, C.H., Y.H. Lee and G.Y. Yeom, "The study of atmospheric pressure plasma for surface cleaning," Surface and Coating Technology, 171, 237-240 (2003). [ Links ]
Received: June 15, 2010.
Accepted: February 11, 2011.
Recommended by Subject Editor Orlando Alfano.














