Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Latin American applied research
versión impresa ISSN 0327-0793versión On-line ISSN 1851-8796
Lat. Am. appl. res. vol.42 no.1 Bahía Blanca ene. 2012
Flotation of manganese minerals and quartz by sodium oleate and water glass
E. M. Andrade, B. L. C. M. Costa, G. A. G. Alcântara and R. M. F. Lima
Mining Engineering Department, Universidade Federal de Ouro Preto, Campus Universitário do Morro do Cruzeiro, S/N., CEP.: 35.400-000 -Ouro Preto- Minas Gerais, Brasil. rosa@demin.ufop.br
Abstract— In this paper, we present the results and a discussion of microflotation tests performed with manganese minerals (rhodonite and rhodochrosite) and quartz mineral, using sodium oleate as the collector. We also explore the influence of water glass (sodium silicate) as a depressant in the recovery of these minerals with sodium oleate. Potential zeta determinations were carried out with the three minerals conditioned with deionised water and with the previously mentioned reagents. It was found that, at a pH of 9, the recoveries of rhodonite and quartz were maximised, and for the rodhocrhosite mineral, the maximum recovery was achieved at pH 11. The water glass at pH 9 was more efficient in depressing quartz than rhodonite, especially for smaller concentrations, whereas the flotation response of rhodochrosite was only slightly affected at both pH 9 and pH 11. The potential zeta curves indicate that the IEP of rhodonite (pH=2.8) is similar to values reported in the literature, whereas the IEP values of quartz (pH=2.2) and rhodochrosite (pH=10.8) differ slightly from those in the literature. The zeta potentials of rhodonite, quartz and rhodochrosite in the presence of sodium oleate and water glass become more negative in a basic medium. Finally, based on the experimental results with a collector of sodium oleate and a depressant of water glass, some inferences on the adsorption mechanisms of these reagents on rhodonite, quartz and rhodochrosite surfaces are made.
Keywords— Manganese; Rhodochrosite; Rhodonite; Quartz; Water Glass..
I. INTRODUCTION
The State of Minas Gerais was the first to exploit manganese-oxide-rich ore in Brazil. Until the 1970s, Minas Gerais supplied the national metallurgy industry and was responsible for almost all exported Brazilian manganese ore products for foreign markets. Despite the exhaustion of manganese-rich deposits, Minas Gerais still has large manganese-poor deposits (queluzite and gondite). These ores have a complex mineralogy consisting of several manganese minerals, such as oxide, silicate and carbonate, that are associated with gangue minerals such as quartz and other silicates, sulphides, carbonates and other minerals.
Acevedo (1977) studied the possibility to concentrate, by flotation, the silicate-carbonate manganese-poor ore from the State of Minas Gerais. For the first step, microflotation tests in a Hallimond cell with pure minerals (rhodonite, rhodochrosite) using sodium oleate and several depressants were performed. It was found that the pH values of maximum recovery for rhodonite were 9 and 10 at an oleate dosage of 5.0 x 10-4 M. The rhodochrosite recovery was constant and maximum (100%) for all pH values studied. It is likely that the reagent concentration used was very high. Furthermore, the floatability of rhodonite, preconditioned with water glass, decreased by 15% at pH 10. In the case of rhodochrosite, it was found that the water glass did not have any depressant effects on the floatability of this mineral. However, in bench tests carried out with manganese ore fraction sizes between 43 and 106 µm, no selectivity between the manganese and gangue minerals was observed.
Lima et al. (2008) performed bench flotation studies, at pH 11 by using sodium oleate as a collector and water glass as a depressant, of silicate-carbonate manganese tailings (Mn, SiO2, Al2O3 and Fe contents of 28.30, 28.10, 9,30 and 3.67%, respectively) from Morro da Mina Mine, located in Conselheiro Lafaiete, Minas Gerais, Brazil. They obtained a Mn recovery of 63%, and the contents of SiO2 and Mn in the concentrate were 17 and 32%, respectively. Despite a reduction of 11% in the SiO2 content in the concentrate as compared with the feed, the Mn content in the concentrate increased by only about 4%. What can be related with the high proportions of manganese silicate minerals such as spessartite, rhodonite and tephroite in their sample (Lima et al., 2010).
Somasundaran (apud Fuerstenau et al., 1985) used diagrams of species distributions for a sodium oleate concentration of 3 x 10-5M and concluded that, at pH 7.8, the ionomolecular specimen (RCOOH.ROO-) activity is maximised and the ionic oleate (RCOO-) and dimer (RCOO22-) activities increase as the pH is increased up to pH 7.8. Above this pH value, both (RCOO- and RCOO22-) activities remain constant. For pH values lower than 7.8, neutral oleic acid begins to precipitate.
The dissociation of water glass (modulus 1) in water produces Na+, H2SiO42-. Furthermore, as a function of pH, the anion H2SiO42- hydrolyses in two steps to H3SiO4- and H4SiO4 (Grenburg and Sinclair; apud Misha, 1982). At pH values below 9, the predominant specimen in the solution is silicic acid [H4SiO4]. In this region, there is a smaller quantity of [SiO(OH)3-], which is predominant at pH values between 9.5 and 12.5. At pH values greater than 6, the specimen [SiO2(OH)22-] appears, which is then predominant above pH 13. The specimen [Si4O6(OH)62-] does not predominate at any pH value, but its concentration does reach a maximum between a pH of 10 and 12; see Figure 1 (Marinakis and Shergold, 1985).
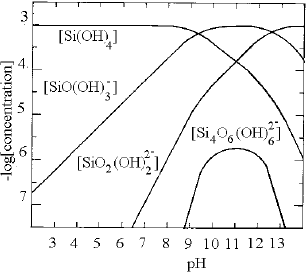
Figure 1. Logarithmic concentration diagram for 10-3 molL-1 SiO2 solution (Marinaks and Shergold, 1985).
In this paper, we present the results of microflotation tests and zeta potential determinations of two manganese minerals, rhodochrosite (MnCO3) and rhodonite ((Mn,Fe,Mg,Ca)SiO3), as well as quartz (SiO2), with the objective of determining the pH and oleate concentration at which maximum recoveries are obtained. We also investigate the influence of the previous conditioning of these minerals with water glass.
II. METHODS
A. Minerals and reagents
Natural quartz (SiO2) sand and rhodonite ((Mn,Fe,Mg,Ca)5(SiO3)5) crystals from Quadrilátero Ferrífero and Morro da Mina Mine, respectively, and a synthetic sample of rhodochrosite (manganese carbonate, MnCO3.XH2O) manufactured by VETEC were used for the microflotation tests and zeta potential determinations.
Table 1 presents the chemical compositions of the pure minerals (natural and synthetic). As can be observed, the MnO content (41.06%) of the rhodonite from Morro da Mina Mine is smaller than the published MnO (49.44%) value. The contents of FeO (3.96%) and MgO (1.06%) are higher than the published stoichiometric values of rhodonite (FeO - 1.11%; MgO - 0.620%), which may be due to the isomorphic substitution of Mn2+ by Fe2+ and Mg2+ in the crystalline lattice of the mineral (Webmineral.com, 2009). The quartz sample has traces of aluminium, iron and potassium, which is in agreement with literature data (Dana, 1984). In this sample, MgO and CaO impurities were also detected. The difference in the rhodochrosite content (approx. 62%, not 100%) is due to the loss of ignition.
Table 1 - Chemical composition of rhodochrosite, rhodonite and quartz minerals. 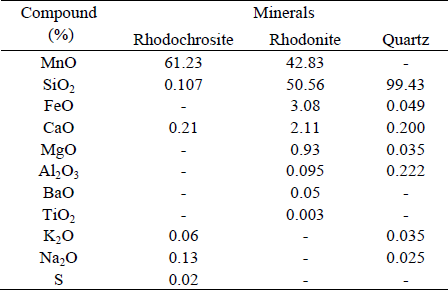
Analytical-grade reagents were used in the flotation studies as follows: oleic acid (C18H34O2) manufactured by Dinâmica, used as a collector; water glass (Na2SiO3. X H2O) manufactured by ISOFAR (Na2O min. of 22% and SiO2 min. of 20%), used as a depressant and a pH regulator; HCl manufactured by VETEC; and NaOH manufactured by Dinâmica. NaOH was also used to prepare the oleate sodium soap.
B. Experimental techniques
The quartz and rhodonite crystals were crushed in a porcelain mill and screened to give a fraction size between 43 and 106 µm for the microflotation tests. The artificial rhodochrosite was used without size classification. The quartz and rhodonite fractions below 37 µm and the synthetic rhodochrosite were crushed in an agate mortar to give fractions (90% smaller than 10 µm) for zeta potential determinations, performed by a Molvern Zetasizer Nano Z - ZEN 2600 device in which the samples were in a 0.5% w/w suspension with or without reagents at several pH values.
A solution of sodium oleate was prepared by diluting 1 g of oleic acid in a 50-mL beaker with constant stirring and adding a 1.7 mL of sodium hydroxide solution at a 10% w/v concentration. The solution was continuously stirred until a limpid and yellow-coloured solution was obtained. Finally, the solution was transferred to a 100-mL volumetric balloon, and the volume was brought to the mark with distilled water to obtain a concentration of 1% w/v.
The water glass solution (1% w/v) was prepared by diluting 1 g of water glass with distilled water in a 100-mL volumetric balloon.
The microflotation tests (three replicates) were performed using a modified Hallimond cell equipped with a magnetic stirrer. Initially, about 1 g of mineral sample was conditioned at the desired pH (pH 4-12) for 2 minutes for several oleate concentrations. First, the pH and oleate concentrations for the highest recovery for each mineral were determined. Furthermore, depression studies were performed for each mineral; the minerals were previously conditioned with varying water glass concentrations for 3 minutes followed by 2 minutes of conditioning with the previously determined optimal oleate concentration. After conditioning, the mineral sample was floated for 1 minute using commercial nitrogen (flow of 60 mL/min.).
III. RESULTS
Figures 2 to 4 present the effects of the oleate concentration and pH on the mineral sample recoveries. The maximum recovery for rhodochrosite occurred at a pH value of 11 (Fig. 2). For rhodonite (Fig. 3) and quartz (Fig. 4), it occurred at a pH value 9. The pH ranges of maximum recovery for both rhodonite and quartz increased with increasing oleate concentration. However, a recovery decrease for all three minerals was observed at a pH of 12.
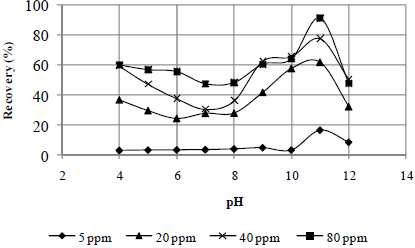
Fig. 2. Effect of pH and sodium oleate concentration on rhodochrosite recovery.
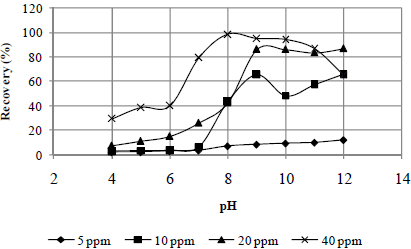
Fig. 3. Effect of pH and sodium oleate concentration on rhodonite recovery.
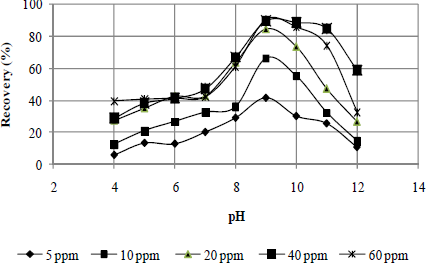
Fig. 4. Effect of pH and sodium oleate concentration on quartz recovery.
In general, it can be affirmed that the sodium oleate had a high affinity for all three studied minerals and that it is not possible to obtain a selective separation among them by flotation with this collector without a depressant, especially in the case of rhodonite and quartz, whose maximum recovery values occurred at a pH value of 9 (Fig. 3 - 4). In the case of rhodochrosite, the greatest recovery occurred at a pH value of 11 (Fig. 2).
Figures 5 and 6 present the effect of water glass on the recoveries of quartz, rhodonite and rhodochrosite at the previously determined sodium oleate concentration corresponding to a maximum recovery: 40 ppm for quartz and rhodonite and 80 ppm for rhodochrosite at pH values of 9 and 11, respectively.
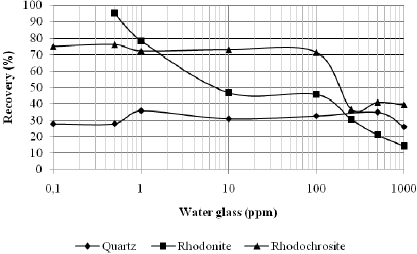
Fig. 5. Recoveries of quartz, rhodonite and rhodochrosite with sodium oleate, preconditioned with water glass at a pH value of 9.
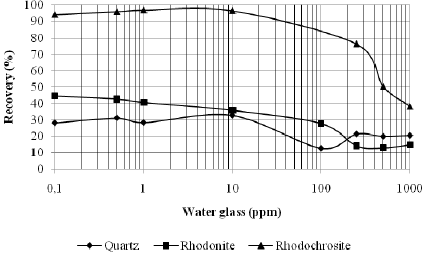
Fig. 6. Recoveries of the minerals quartz, rhodonite and rhodochrosite with sodium oleate, preconditioned with water glass at a pH value of 11.
At both pH values studied, the water glass was found to be more efficient in depressing quartz than rhodonite, especially for concentrations smaller than 100 ppm. The opposite trend was observed for rhodochrosite; see Fig. 5 and 6. Thus, the selective separation of manganese minerals (rhodochrosite and rhodonite)from quartz mineral is dependent on the water glass concentration.
For both pH values (9 and 11), the water glass was not efficient in depressing the rhodochrosite mineral up to a concentration of 100 ppm; see Fig. 5 and 6. This means that, for manganese ore that contains carbonate manganese (rhodochrosite), silicate manganese (rhodo-nite) with quartz could be possible to produce concentrates with lower SiO2 contents by flotation with water glass as a depresssant and sodium oleate as a collector. However, the system under study was very simple. It would be valuable to study the influence of a hidroxy complex of Mn in the solution on silicate mineral recovery with water glass and sodium oleate, once rhodochrosite is a semi-soluble mineral.
The isoeletric point for rhodochrosite occurred at a pH value of 10.4. This value differs from the value of 7.2 determined by Song and Lu (1994) and 4.8 of a natural Egyptian rhodochrosite sample that was determined by the streaming potential method (Abeidu, 1973); see the zeta potential curve of the mineral determined without reagents (Fig. 7).
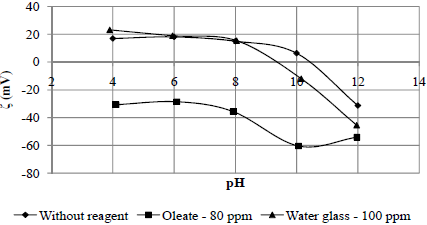
Fig. 7. Zeta potential of rhodochrosite conditioned without and with reagents.
In general, for rhodochrosite conditioned with sodium oleate, the zeta potentials became negative for pH values below the isoeletric point (pH 10.4) and decreased with increasing pH, except for the pH value of 12 (Fig. 7) when compared with the potential zeta of the mineral in the absence of reagents. This behaviour is in accordance with the lower recovery obtained at a pH value of 12 pH in the microflotation test results presented in Fig. 2. Thus, it can be affirmed that the anionic specimens of oleate present in the solution specifically adsorb on the mineral surface.
As shown by the zeta potential curve of rhodochrosite mineral conditioned with 100 ppm of water glass, the zeta potential values of the mineral were nearly constant up to a pH of 8. Above this value, the zeta potential values became more negative as compared with the values of the mineral without reagent. This behaviour could be attributed to specific adsorption of the specimens [SiO(OH)3-], [SiO2(OH)22-] and [Si4O6(OH)62-] present in the solution, especially for pH values above 9, as reported by Marinakis and Sherhold (1985); see Fig. 7.
The isoeletric point (pH 2.8) of the rhodonite was the same as that reported by Fuerstenau et al. (1976) (Fig. 8). After the mineral came into contact with sodium oleate, the zeta potentials at all of the pH values were negative and became more negative as the pH values increased. As in the case of rhodochrosite, this behaviour is coherent with the microflotation tests, which means that recovery increases with increasing pH value due to increased specific adsorption of the anionic specimens of the collector on the mineral surface. The point at which the zeta potential values became more negative with pH corresponds to the point of maximum recovery.
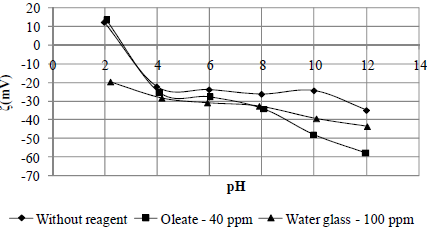
Fig. 8. Zeta potential of rhodonite conditioned with and without reagents.
For rhodonite conditioned with water glass, it was found that, up to a pH value of 6, the zeta potential values were very similar to those obtained for the mineral conditioned without a reagent (Fig. 8). This occurred because, until this pH value is reached, silicic acid (H4SiO4) is the dominate specimen in the solution, and above this pH, H3SiO4- (predominant at a pH range of 9.5 to 12.5), and [SiO2(OH)22-] and [Si4O6(OH)62-] between pH values of 9.5 and 12.5 are present in the solution (Marinakis and Sherhold, 1985), which specifically adsorbed on the mineral surface.
In spite of sample impurities (Table 1), the measured isoeletric point (pH 2.2) of quartz was close to the value reported (pH 1.8) by Iwasaki et al. (1960) (Fig. 9). As in both previous cases (rhodochrosite and rhodonite), the zeta potential values obtained with the mineral conditioned with sodium oleate and water glass were more negative than the zeta potential values of the mineral conditioned without reagent for all pH values, which can be attributed to specific adsorption of the anionic specimens present in the solution on the mineral suface. This behaviour is in accord with the microflotation test results (Fig. 6 and 7).
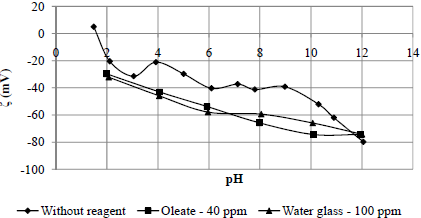
Fig. 9. Zeta potential of quartz conditioned with and without reagents.
It was found that the water glass was more efficient in depressing quartz, followed by rhodonite. This efficiency was higher at pH 9 as compared with pH 11 at concentrations smaller than 100 ppm (see Fig. 5). This means that it may be possible to achieve selectivity between these two minerals by flotation, especially at these pH values, due to the previous adsorption of anionic specimens of water glass present in the solution, which could be attributed to hydrogen bonding between the hydroxyl group on the mineral surfaces, especially for quartz, and the anionic specimens present in the solution (H3SiO4-), which prevent the adsorption of anionic specimens of oleate present in the solution in pH values of 9 and 11, respectively.
IV. CONCLUSIONS
Based on the above studies carried out with manganese minerals and quartz, which are present in silicate-carbonate manganese ore from Morro da Mina Mine, it was concluded that:
- The maximum recovery of quartz and rhodonite minerals occurred at a pH value of 9, and for synthetic rhodochrosite, it occurred at pH 11.
- Water glass was more efficient in depressing quartz than rhodonite for dosages smaller than 100 ppm with 40 ppm of sodium oleate at pH values of 9 and 11.
- Rhodochrosite recovery decreased by about 15% for water glass concentrations ranging from 0.1 to 100 ppm at a pH value of 11 and a sodium oleate concentration of 80 ppm.
- The isoelectric points of quartz, rhodonite and rhodochrosite were found to be: 2.2; 2.7 and 10.4, respectively.
- All zeta potential values of the three studied minerals conditioned with sodium oleate were smaller than those values determined without a reagent. Thus, the adsorption mechanism of oleate on the surface of all studied minerals is specific.
- The zeta potential values of quartz, rhodonite and rhodochrosite conditioned with water glass up to a pH of 6 were very similar to those obtained by conditioning with distilled water. The values also became more negative for pH values above 6.
- The dominant specimen present in the solution at pH values from 9 to 11 is H3SiO4-. Thus, this specimen specifically adsorbed on the negatively charged surfaces of the studied minerals.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge FAPEMIG for its financial support of the project and for a scholarship (G. A.G. Alcântara); the Morro da Mina Mine for the rhodonite; CAPES for the scholarship for the master student (E. M. Andrade); and CNPq for a scholarship (B. L. C. M., Costa).
REFERENCES
1. Abeidu, A.M., "The Feasibility of Activation of Manganese Minerals Flotation," Trans. JIM, 14, 45-49 (1973). [ Links ]
2. Acevedo, G.S., "Flotation of silicate-carbonated manganese ores using oleic acid and depressors," Avances en flotación, S. Castro e J. Alvarez (Ed). Universidad de Concepción, 3, 50-60 (1977). [ Links ]
3. Dana, J. D., Manual de Mineralogia; review by C. Hurlbult Jr., traslation by R.R. Franco, Rio de Janeiro, Livros Técnicos e Científicos Editora S.A. (1984). [ Links ]
4. Fuerstenau, M.C. (ed) Flotation: A. M. Gaudin Memorial Volume. New York: American Institute of Mining, Metallurgical and Petroleum Engineers (1976). [ Links ]
5. Fuerstenau, M.C., J.D. Miller and M.C. Kuhn, Chemistry of Flotation, SME. Tennessee New York (1985). [ Links ]
6. Iwasaki, I., S.R.B. Cooke and A.F. Colombo, Flotation Characteristics of Goethite, Washington United State of Interior, Berau of Mines, Report of investigation 5593 (1960). [ Links ]
7. Lima, R.M.F., J.A. Vasconcelos and G.R. Silva, "Flotação Aniônica de Minério de Manganês," Revista da Escola de Minas, 61, 337-342 (2008). [ Links ]
8. Lima, R.M.F., E.E. Pereira, E.L. Reis and G.R. Silva, "Caracterização e Concentração de Rejeito de Minério de Manganês," Proc. 65° Congresso da ABM, Rio de Janeiro, Brasil, 116-1125 (2010). [ Links ]
9. Marinakis, K.I. and H.L. Shergold, "Influence of Sodium Silicate addition on the Adsorption of Oleic Acid by Fluorite, Calcite and Barite," International Journal of Mineral Processing, 14, 177-193 (1985). [ Links ]
10. Misha, S.K.. "Eletrokinetic Properties and Flotation Behavior of Apatite and Calcite in the Presence of Sodium Oleate and Sodim Metasilicate," International Journal of Mineral Processing, B, 59-73 (1982). [ Links ]
11. Song, S. and S. Lu, "Hydrophobic Flocculation of Fine Hematite, Siderite and Rhodochrosite Particles in Aqueous Solution," Journal of Colloid and Interface Science, 166, 73-80 (1994). [ Links ]
12. Webmireral.com, http:// Webmineral.com. Accessed in august, 08 (2009). [ Links ]
Received: October 21, 2010.
Accepted: April 19, 2011.
Recommended by Subject Editor Ricardo Gómez.














