Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Latin American applied research
versión impresa ISSN 0327-0793versión On-line ISSN 1851-8796
Lat. Am. appl. res. vol.42 no.1 Bahía Blanca ene. 2012
Removal of Direct Black 38 dye by adsorption and photocatalytic degradation on TiO2 prepared at low temperature
G. C. Collazzo*†, S. L. Jahn† and E. L. Foletto†
† Chemical Eng. Department, Federal University of Santa Maria, Santa Maria-RS, 97105-900, Brazil.
gabicollazzo@yahoo.com.br
Abstract— TiO2 powder was prepared by a hydrothermal process at 200°C for 12 h. The material was characterized by X-ray diffraction and N2 adsorption-desorption isotherm. The synthesized sample presented a pure phase anatase, with nanometric particle size. Its activity was tested for adsorption and degradation of the azo leather dye, Direct Black 38. The adsorption isotherm obtained followed the S-type in terms of the classification of Giles. Thermodynamic parameters were calculated, and the results revealed that the adsorption process is endothermic in nature. The material showed highly photocatalytic activity in the degradation of the dye, similar to that of the TiO2 (P25 Degussa) photocatalyst.
Keywords— TiO2; Photocatalysis; Adsorption; Degradation; Dye.
I. INTRODUCTION
Dye effluents may lead to certain environmental problems. Effluent treatment for dye-containing effluents from tanneries and textile and paper industries is currently capable of removing only about half of the dyes lost in residual liquors (Moreira et al., 2005). Advanced oxidation processes (AOPs) have been proposed as an alternative method for water purification and an efficient wastewater treatment technique used for the total mineralization of organics (Akyol et al., 2004). AOPs include ozonation, UV irradiation, photocatalysis, the Fenton and photo-Fenton reagent, and various combinations of these (Tarek et al., 2009).
The photocatalytic degradation of toxic compounds in aqueous media provides a new method for wastewater treatment. The process couples low-energy ultraviolet light with semiconductors acting as photocatalysts. TiO2 is generally used as a photocatalyst for the removal of organic pollutants (Tanaka et al., 1997) and dye pollutants (So et al., 2002) due to its high photocatalytic activity and stability, low cost, stability with respect to corrosion, and biological, nontoxic and chemical inertness (Ferguson et al., 2005).
Different methods have been employed for the synthesis of titanium dioxide, as follow: chemical precipitation (Scolan and Sanchez, 1998), sol-gel method (Lin et al., 2006a), hydrothermal (Wu et al., 2002) and solvothermal processes (Yin et al., 2003). Hydrothermal synthesis has become one of the notable methods employed in nano-material production and nanotechnology (Pavasupree et al., 2008). The process includes a simple route and produces high crystallinity oxides under moderate conditions: low temperatures and short reaction times. It is one the most used methods to produce ceramic materials as it allows the control of particle size, morphology (Lin et al., 2006a) and phase composition (Wang et al., 2007).
In this study, TiO2 was synthesized by the hydrothermal method at 200°C for 12 h. Its characterization, adsorption capacity and photocatalytic activity in the degradation of azo leather dye, Direct Black 38, was investigated. Various thermodynamic parameters, such as entropy (ΔS°), heat of adsorption (ΔH°) and Gibbs free energy (ΔG°) have been determined.
II. METHODS
A. Catalyst preparation
The synthesis of titanium dioxide was performed using hydrothermal treatment. The molar composition of the reaction mixture was 1TiO2:10CH3COOH:150H2O. Both glacial acetic acid (CH3COOH) (Aldrich) and titanium (IV) isopropoxide (C12H28O4Ti) (Aldrich) were of analytical-reagent grade and used as starting materials. Glacial acetic acid (36 mL) was slowly added to 20 mL of titanium (IV) isopropoxide in a water bath at 0°C, under constant stirring. Afterwards, deionized water (170 mL) was gently added to the mixture under constant stirring. The solution was then mixed together under vigorous agitation for one hour and subsequently underwent ultrasonic treatment for 30 min. Once again, vigorous agitation was applied for 5 h. Afterwards, the solution was poured into teflon jars and placed in stainless steel autoclaves. The autoclaves were placed in an oven previously heated to 70°C in order to carry out the ageing process for 12 h. Following the ageing process, hydrothermal treatment was carried out 200°C for 12 h. Subsequently, the autoclaves were removed from the oven and cooled in running water. Eventually, the precipitate was washed with distilled water, centrifuged and dried in an oven at 100°C for 12 h. The solid sample obtained was finely ground using a mortar and pestle and stored in plastic containers.
B. Characterization methods
The powder was characterized by X-ray diffraction and nitrogen adsorption was determined by BET. X-ray diffraction (XRD) patterns were obtained using a Bruker D8 Advance diffractometer with a Cu-Ka radiation X-ray source (40 kV e 40 mA). Data were collected over the 2Δ range 20-80° with a step size of 0.05° and a count time of 35 s. The average nanocrystal size was estimated using the Sherrer equation. The Brunauer-Emmett-Teller (BET) surface area measurements were carried out by N2 adsorption-desorption at 77 K using a Quantachrome Autosorb Automated Gas Sorption instrument, in the relative pressure range (P/Po) of 0 to 0.99.
C. Adsorption Study
For the adsorption tests, 0.2 g of TiO2 was added to 200 mL of the aqueous solution of azodye Direct Black 38 (DB38) at different initial concentrations and at optimum pH 2.5, which was adjusted by using dilute H2SO4. The pH 2.5 was determined by previous studies conducted by Moreira et al. (2005) as the optimal value. The resulting solution was stirred continuously using a magnetic stirrer (100 rpm) at a constant temperature of 25°C to achieve the adsorption equilibrium of dye on the catalyst. An aliquot of the aqueous solution was taken at various time intervals, centrifuged and filtered through a PVDF membrane (0.22 μm) before analysis. The concentration of dye in aqueous solution was determined by spectrometry (Spectro vision model T6-UV) at lmáx 590 nm. UV-vis adsorption spectrum of DB38 aqueous solution at pH 2.5 was shown in a previous study (Moreira et al., 2005).
D. Photocatalysis Study
The photocatalytic activity of the commercially available TiO2 Degussa P25 was also evaluated and compared with that of TiO2. TiO2 Degussa P25 is mostly in the anatase form (80% anatase form and 20% rutile) and has a BET surface area of 50 m2.g-1 corresponding to a mean particle size of 30 nm, and mean pore diameter of about 6.9 nm.
The reactor was batch-type, consisting of a glass tube (internal diameter of 7.5 cm and 18.0 cm in height) with a mercury vapor lamp (80 W) fixed at the center and protected by a bulb of quartz. The outside of the reactor was covered with aluminum foil to protect from external UV radiation, so that the rays were reflected into the reactor. Furthermore, the reactor was equipped with an aluminum cylinder to keep the temperature constant. The photocatalytic degradation of dye DB38 was studied for different initial concentrations of dye and different concentrations of catalyst, at pH 2.5, 25°C and a fixed volume of 400 mL of solution. Prior to photoirradiation, the suspensions underwent ultrasound for 10 min. Before illumination, the suspension with DB38 and photocatalyst was magnetically stirred for 60 min to establish the adsorption equilibrium. An initial sample was taken out for analysis at the end of the adsorption period, in order to determine the bulk concentration. This was considered as the initial concentration (hereafter denoted Co) for the photocatalytic experiment from which the rate constants were determined.
The mercury vapor lamp was turned on and the solution was stirred (100 rpm) using a magnetic stirrer and aliquots were taken at certain periods of time. These samples were centrifuged and filtered with a PVDF membrane (0.22 nm) before the analysis of color. The degradation of the dye was monitored by measuring the absorbance at 590 nm until the process reached the equilibrium.
III. RESULTS AND DISCUSSION
A. Characterization of TiO2
Figure 1 (a) shows XRD for the TiO2 sample synthesized. The diffractogram shows the complete formation of the crystalline anatase phase, indexed according to JCPDS card No. 89-4921. The Fig. 1 (b) shows the isotherm adsorption/desorption of nitrogen for the titanium dioxide synthesized. The curve shows a type IV isotherm and its hysteresis loops, typical of mesoporous materials according to the recommendations of IUPAC (International Union of Pure and Applied Chemistry). The crystalline size, surface area, and total pore volume of the resulting sample were 13 nm, 113 m2.g-1and 0.31cm3.g-1, respectively.

Fig. 1. (a) XRD of the TiO2 sample synthesized and (b) N2 adsorption-desorption isotherm.
B. Considerations on adsorption
Figure 2 shows the kinetics of dye adsorption on TiO2 at different initial dye concentrations. The experimental conditions were: Ccatalyst = 1 g.L-1, pH = 2.5, temperature = 25°C. Observe that for all concentrations, the equilibrium is reached in the first 10 min. This is due to the fact that the material synthesized has a high surface area, thus providing many sites available for adsorption, resulting in an increase in the concentration gradient of adsorbate to adsorbent surface. This increase in the dye concentration gradient tends to increase the adsorption of the dye in the initial stage.

Fig. 2: Kinetic of adsorption of DB38 on TiO2 with different initial dye concentrations.
The analysis of the adsorption process requires a study of adsorption equilibrium. The adsorption equilibrium test provides physico-chemical data for the applicability of adsorption. The two most commonly used equilibrium relations are Freundlich and Langmuir isotherm equations. The equation describing the Langmuir isotherm is given by (Vadivelan and Kumar, 2005):
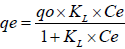 | (1) |
where qo is the maximum amount of adsorption corresponding to complete monolayer coverage on the surface (mg.g-1), KL is the Langmuir constant related to the energy of adsorption (L.mg-1). The Langmuir constants qo and KL were calculated from the slope of the plot between 1/qe versus 1/Ce (not shown). Through this plot 1/qe versus 1/Ce, the values of these parameters to fit the experimental data were qo of 122 mg.g-1, KL of 0.019 L.mg-1 and with a correlation coefficient (r2) of 0.69. Through the low value of r2 note that the inability of the Langmuir model to represent the experimental data could have been due to the fact that this isotherm does not take adsorbate-adsorbate interactions into account. The equation that describes the Freundlich isotherm is given by (Gómez et al., 2007):
 | (2) |
where KF is the adsorption capacity (mg.g-1) and 1/n is the adsorption intensity (L.g-1).
Through the plot log qe versus log Ce (not shown), the values of these parameters to fit the experimental data were KF 1.28 mg.g-1, 1/n of 1.15 L.g-1 with a correlation coefficient (r2) of 0.87. The Freundlich equation provided a more satisfactory description when compared to the Langmuir isotherm.
Using the Giles classification (Giles et al., 1997), the experimental isotherm obtained in present study was the S-type (Fig. 3). The initial part of the S curve indicates few interactions between dye and the solid at low concentrations. However, as the concentration in the liquid phase increased, adsorption occurred more readily. This behavior is due to a synergistic effect, with the adsorbed molecules facilitating the adsorption of additional molecules as a result of attractive adsorbate-adsorbate interactions. Gómez et al. (2007) and G ürses et al. (2006) found S-type isotherms for adsorption of various dyes on activated carbon.

Fig. 3: Isotherm of DB38 adsorption on TiO2.
The study of thermodynamics of adsorption was carried out at a temperature ranging from 25 to 55°C in order to determine DG°, DH° and DS°. The Gibbs free energy of the adsorption process is related to the equilibrium constant by the classical Van't Hoff equation (Uma et al., 2009).
 | (3) |
The Gibbs free energy change is also related to the entropy change and heat of adsorption at constant temperature according to the following equation:
 | (4) |
Combining the above equations:
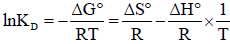 | (5) |
where ΔG° is the free energy change (J.mol-1), ΔH° the change in enthalpy (J.mol-1), ΔS° the entropy change
(J.mol-1.K-1), T is the absolute temperature (K), R is the universal gas constant (8.314×10−3 KJ.mol-1.K-1) and KD (qe/Ce) is the single point or linear adsorption distribution coefficient.
The ΔH° can be determined by the slope of Van't Hoff equation, ln KD versus 1/T using the equation:
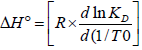 | (6) |
Table 1 shows the values found for the thermodynamics parameters. The positive value for enthalpy change means that the adsorption of this compound is endothermic, i.e, the removal of dye is favored by increasing temperature. As the value obtained for enthalpy was 560 J.mol-1, or that is, less than 40.000 J.mol-1 (Sankar et al., 1999) with weak forces of Van der Waals (Wedler, 1976) has been the characterization of a physical nature of adsorption. The positive value of ΔS° suggest increased randmmess at the solid/solution interface with the increased of temperature. As for the variation of Gibbs free energy, positive values indicate that the enthalpic contribution is higher than the entropic contribution. It is observed that the variation of Gibbs free energy decreases with increasing temperature, showing that the adsorption is more favorable at higher temperatures.
Table 1: Thermodynamics parameters of the adsorption of dye on titanium dioxide (Co=200mg.L-1, Cadsoben =1 g.L-1, pH=2.5)

C. Considerations on photocatalysis
The degradation of DB38 has been investigated to determine whether degradation occurred when the dye solution was irradiated with UV in the presence of TiO2 (P25-Degussa) and TiO2 synthesized by hydrothermal suspension. Figure 4 shows the photocatalytic degradation of DB38 as a function of irradiation time for both photocatalysts. The photoactivity of TiO2 was similar to that of TiO2-P25, which may be attributed to its great specific surface area (113 m2.g-1), high crystallinity and nanometric particle size (13 nm). It is interesting to note that TiO2 and P25 exhibit similar activity despite have different properties such as crystalline phase composition, particle size and BET area. The P25 powder is manufactured by the Aerosil process, by which titanium tetrachloride is subjected to hydrolysis in vapor phase at an elevated temperature (Bickley et al., 1991). Different preparation methods have been employed for the two samples and this could affect properties such as active site density, aggregate particle size in solution and other nano-structural parameters and consequently alter reactivity (Gumy et al., 2006). The anatase phase of TiO2 is considered the more active (Linsebigler et al., 1995), however, the mixed structure of 80/20 anatase/rutile (P25) may cause a synergy effect which must be the key to the high activity of the P25 (Ohno et al., 2001).
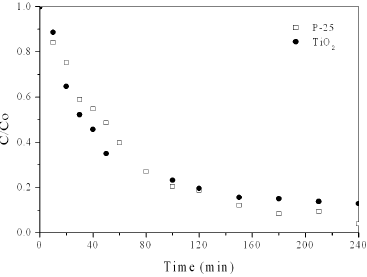
Fig. 4: Photocatalytic degradation of DB38 as a function of time irradiation. Experimental conditions: Co = 100 mg.L-1, Ccatalyst = 1 g.L-1, pH = 2.5, T = 25°C.
The effect of initial DB38 concentration on the rate of degradation was investigated, varying the concentration from 25 to 95 mg.L-1, and leaving the concentration of TiO2 constant at 1.0 g.L-1. Figure 5 shows the effect of the initial dye concentration on the photocatalytic degradation of DB38 and the influence of photolysis on the degradation process. As seen in Fig. 5, there is a negligible influence of photolysis on the degradation of the dye. Note that by increasing the concentration of dye, the rate of degradation decreases. Similar results have been presented for the degradation of other dyes (Wang, 2007). Ishiki et al. (2005) reported that this can be justified by the number of active sites at the TiO2/H2O interface. Thus, at a lower concentration of dye, there are many more water molecules that will be adsorbed on the available TiO2 particles, producing hydroxyl radicals and leading to a rapid oxidation process. In contrast, at higher concentrations of dye there is a smaller proportion of adsorbed water molecules on the catalyst surface because the number of available active sites remains the same. Consequently, competitive adsorption between the dye and water molecules increases and leads to a decrease of the rate constant. The other possible cause is the interference of intermediates formed upon the degradation of dye (So et al., 2002).

Fig. 5: Effect of initial concentration on the photocatalytic degradation of DB38 for different initial dye concentrations. Experimental conditions: Ccatalyst = 1 g.L-1, pH = 2.5, T = 25°C
The initial degradation rate of most organic pollutants is described by the Langmuir Hinshelwood (L-H) model, which is accepted by a great number of re searchers (Konstantinou and Albanis, 2004). It basicallyrelates the degradation rate (r) and reactant concentration in water (C) at time t, which is expressed as follows:
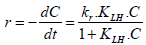 | (7) |
where r is the degradation rate of the reactant (mg L-1.min-1), C the concentration of the reactant (mg. L-1), t the illumination time (min), kr the constant of reaction rate (mg.L-1.min-1), KLH the adsorption equilibrium constant of the reactant (L.mg-1). When the adsorption is relatively weak and/or the reactant concentration is low, Eq. (7) can be simplified to the pseudo-first-order kinetics with an apparent first-order rate constant (kapp):
 | (8) |
where Co is the initial concentration (mg.L-1), kapp is the apparent constant rate of degradation (min-1) and kr.KLH= kapp. The initial degradation rate could be then deducted from Eq. (9):
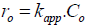 | (9) |
The plot ln (Co/C) versus time in Fig. 6 represents a straight line. The slope of linear regression is equal to the apparent first-order rate constant kapp. Their values corresponding to different initial concentrations are in Table 2. The observed decrease in kapp as the initial concentration of dye increases is explained by the lower availability of photons as the color of the solution gets deeper. The presumed reason is that at high dye concentrations the generation of •OH radical on the surface of the catalyst is reduced since the active sites are covered by dye ions (Konstantinou and Albanis, 2004). At a high dye concentration, a significant amount of UV may be absorbed by the dye molecules rather than the TiO2 particles, which reduces the efficiency of the catalytic

Fig.6: Plot of ln (Co/C) as a function of time irradiation. Experimental conditions: Ccatalyst = 1 g.L-1, pH = 2.5, T = 25°C.
Table 2: Values of kapp and corresponding ro

reaction because the concentrations of •OH decrease (So et al., 2002).
The linear form of the Langmuir-Hinshelwood model, in the following expression, translates the dependence of Co/ro values for the respective Co values of dye concentration.
 | (10) |
Through the plot Co/ro versus Co (not shown), the values of kr and KLH are determined. The kr and KLH values calculated from the slope of the straight line (r2 = 0.97) and from the ordinate were 0.953 mg.L-1.min-1 and 0.042 L.mg-1, respectively. As can be seen, KL (0.019 L.mg-1) deducted from the Langmuir isotherm is smaller that KLH deducted from photocatalytic degradation. According to the Langmuir-Hinshelwood model, if KLH truly reflected the adsorption affinity of the dye on the TiO2 surface, KL and KLH would be identical. However, in other studies, it was reported that KLH could be substantially larger than KL, 280 times for metobromuron, 13 times for benzylic alcohol, 220 times for 4-chlorophenol (Vulliet et al., 2003). Various explanations have been suggested: (i) a photoadsorption could occur (Lin et al., 2006b) and (ii) the reaction could take place not only at the surface but also in the bulk solution (Vulliet et al., 2003).
The effect of catalyst loading on degradation efficiency was investigated by varying the amount of catalysts ranging from 0.25 to 1.00 g.L-1. The initial concentration of DB38 was kept constant during these experiments (100 mg.L-1). Figure 7 shows the influence of the amount of TiO2 in the reactor on the photodegradation rate of DB38. An increase in the rate of degradation was observed with increasing amounts of catalyst as the number of photons absorbed and the number of molecules adsorbed also increased. However this increase varied slightly, from 0.79 to 1.06 mg.L-1.min-1 when considering the greater variation in the concentration of catalyst (0.25 to 1.00 g.L-1), which proves the use larger quantities of catalyst unnecessary.

Fig. 7: Dependence of initial rate of DB38 degradation on catalyst loading in slurry TiO2 system.
IV. CONCLUSIONS
The TiO2 nano material was prepared successfully through a low temperature hydrothermal process. The synthesized material presented a pure phase anatase, with high surface area and a crystal size on the nanometric scale. An evaluation of dye adsorption using TiO2 showed that that the adsorption isotherm obtained followed the S-type in terms of the Giles classification. Various thermodynamic parameters have been calculated (ΔS°, ΔH° and ΔG°) indicating that the adsorption process is endothermic in nature and nonspontaneous. The oxide produced showed efficient catalytic activity in degrading the azodye in aqueous solution which was similar to that of commercially available TiO2 photocatalyst (P25 Degussa).
REFERENCES
1. Akyol, A., H.C. Yatmaz and M. Bayramoglu, "Photocatalytic decolorization of Remazol Red RR in aqueous ZnO suspensions," Appl. Catal. B: Environ., 54, 19-24 (2004). [ Links ]
2. Bickley, R.I., T. González-Carreño, J.S. Lees, L. Palmisano and R.J.D. Tilley, "A Structural Investigation of Titanium Dioxide Photocatalysts," J. Solid State Chem., 92, 178-190, (1991). [ Links ]
3. Ferguson, M.A., M.R. Hoffmann and J. Hering, "TiO2-Photocatalyzed As (III) Oxidation in Aqueous Suspensions: Reaction Kinetics and Effects of Adsorption," Environ. Sci. Technol., 39, 1880-1886, (2005). [ Links ]
4. Giles, C.H., D. Smith and A. Huitson, "A general treatment and classification of the solute adsorption isotherm. I: Theoretical," J. Colloid Interface Sci., 47, 755-765 (1997). [ Links ]
5. Gómez, V., M.S. Larrechi and M.P. Callao, "Kinetic and adsorption study of acid dye removal using activated carbon," Chemosphere, 69, 1151-1158, (2007). [ Links ]
6. Gumy, D., C. Morais, P. Bowen, C. Pulgarin, S. Giraldo, R. Hajdu and J. Kiwi, "Catalytic activity of commercial of TiO2 powders for the abatement of the bacteria (E-coli) under solar simulated light: Influence of the isoelectric point," Applied Catalysis B-Environmental, 63, 26-84 (2006). [ Links ]
7. Gürses, A., C. Dogar, S. Karaca, M.A. Ikyildiz and R. Bayrak, "Production of granular activated carbon from waste Rosa canina sp. seeds and its adsorption characteristics for dye," J. Hazard. Mater., B131, 254-259 (2006). [ Links ]
8. Ishiki, R.R., H.M. Ishiki and K. Takashima, "Photocatalytic degradation of imazethapyr herbicide at TiO2/H2O interface," Chemosphere, 58, 1464-1469 (2005). [ Links ]
9. Konstantinou, I.K. and T.A. Albanis, "TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: kinetic and mechanistic investigations, A review," Appl. Catal. B: Environ., 49, 1-14 (2004). [ Links ]
10. Lin, K.F., C. Su and Y.H. Lin, "Preparation and characterization of high-surface-area titanium dioxide by sol-gel process," J. Porous Mater., 13, 251-258 (2006a). [ Links ]
11. Lin, H.F., R. Ravikrishna and K.T Valsaraj, "Reusable adsorbents for dilute solution separation. 6. Batch and continuous reactors for the adsorption and degradation of 1,2-dichlorobenzene from dilute wastewater streams using titania as a photocatalyst," Sep. Purif. Technol., 28, 87-102 (2006b). [ Links ]
12. Linsebigler, A.L., G. Lu and J.T. Yates, " Photocatalysis on TiO2 surfaces: principles, mechanisms, and selected results", Chemical Reviews, 95, 735-758 (1995). [ Links ]
13. Moreira, R.F.P.M., T.P. Sauer, L. Casaril and E. Humeres, "Mass transfer and photocatalytic degradation of leather dye using TiO2/UV," J. Appl. Electrochem, 35, 821-829 (2005). [ Links ]
14. Ohno, T., K. Sarukawa, K. Tokieda and M. Matsumura, "Morphology of a TiO2 photocatalyst (Degussa, P-25) consisting of anatase and rutile crystalline phases," J. Catal., 203, 82-86 (2001). [ Links ]
15. Pavasupree, S., J. Jitputti, S. Ngamsinlapasathian and S. Yoshikawa, "Hydrothermal synthesis, characterization, photocatalytic activity and dye-sensitized solar cell performance of mesoporous anatase TiO2 nanopowders," Mater. Res. Bull., 43, 149-157 (2008). [ Links ]
16. Sankar, M., G. Sekaran, S. Sadulla and T. Ramasami, "Removal of diazo and triphenylmetahne dyes from aqueous solutions through and adsorption process," J. Chem. Technol. Biotechnol., 44, 337-344 (1999). [ Links ]
17. Scolan, A. and C. Sanchez, "Synthesis and characterization of surface-protected nanocrystalline titania particles," Chem. Mater., 10, 3217 - 3223 (1998). [ Links ]
18. So, C.M., M.Y. Cheng, J.C. Yu and P.K. Wong, "Degradation of azo dye Procion Red MX-5B by photocatalytic oxidation," Chemosphere, 46, 905-912 (2002). [ Links ]
19. Tanaka, K., W. Luesaiwong and T. Hisanaga, "Photocatalytic degradation of mono-, di- and trinitrophenol in aqueous TiO2 suspension," J. Mol. Catal. A: Chem., 122, 67-74 (1997). [ Links ]
20. Tarek, A., G-A., S. Kato, S. Satokawa and T. Kojima, "Treatment of synthetic dyes wastewater utilizing a magnetically separable photocatalyst (TiO2/SiO2/ Fe3O4): Parametric and kinetic studies," Desalination, 244, 1-11 (2009). [ Links ]
21. Uma, R.L., V.C. Srivastava, I.D. Mall and D.H. Lataye, "Rice husk ash as an effective adsorbent: Evaluation of adsorptive characteristics for Indigo Carmine dye," J. Environ. Manage., 90, 710-720 (2009). [ Links ]
22. Vadivelan V. and K.V. Kumar, "Equilibrium, kinetics, mechanism, and process design for the sorption of methylene blue onto rice husk," J. Colloid Interface Sci., 286, 90-100 (2005). [ Links ]
23. Vulliet, E., J.-M. Chovelon, C. Guillard and J.-M. Herrmann, "Factors influencing the photocatalytic degradation of sulfonylurea herbicides by TiO2 aqueous suspension," J. Photochem. Photobiol., A 159, 71-79 (2003). [ Links ]
24. Wang, G., "Hydrothermal synthesis and photocatalytic activity of nanocrystalline TiO2 powders in ethanol-water mixed solutions," J. Mol. Catal. A: Chem., 274, 185 -191 (2007). [ Links ]
25. Wedler, G., Chemisorption: An Experimental Approach, Butterworths, London (1976). [ Links ]
26. Wu, M., G. Lin, D. Chen, G. Wang, D. He, S. Feng and R. Xu, "Sol-Hydrothermal Synthesis and Hydrothermal Structural Evolution of Nanocrystal Titanium Dioxide," Chem. Mater., 14, 1974-1980 (2002). [ Links ]
27. Yin, S., Y. Fujishiro, J. Wu, M. Aki and T. Sato, "Synthesis and photocatalytic properties of fibrous titania by solvothermal reactions," J. Mater. Process. Technol., 137, 45-48 (2003). [ Links ]
Received: September 26, 2010.
Accepted: April 1, 2011.
Recommended by Subject Editor Orlando Alfano.














