Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Latin American applied research
versión impresa ISSN 0327-0793versión On-line ISSN 1851-8796
Lat. Am. appl. res. vol.42 no.1 Bahía Blanca ene. 2012
Novel optical inmunoassay based on macroporous silicon waveguide for determining hidroxysafflor yellow
X. Y. L܆, Z. H. Jia‡, J. W. Li§ and F. C. Zhang§
† Postdoctoral Station of Computer Science and Technology, Xinjiang University, Urumqi, PR China.
‡ College of Information Science & Engineering, Xinjiang University, Urumqi, PR China.
xiaoz813@yahoo.com.cn
§Key Laboratory of Xinjiang Biological Resources and Gene Engineering, College of Life Sciences and Technology, Xinjiang University, Urumqi, PR China
Abstract— Porous silicon (PSi) has attracted much attention for biosensing due to its large surface area and easy preparation. In this paper, an optical immunoassay method based on a macro-PSi silicon resonant waveguide, with pore size much larger than that of large molecules such as bovine serum albumin, has been developed for the detection of the antigen-antibody reaction between hydroxysafflor yellow A (HSYA, i.e., the component of Carthamus tinctorius L.) and the specificity of the polyclonal anti-HSYA antibodies. HSYA antibodies were immobilized into the macro-PSi silicon waveguide using standard amino-silane and glutaraldehyde chemistry. The waveguide resonance angle was increased by binding HSYA onto the immobilized antibodies. The label-free immunosensor is simple and exhibits high sensitivity to HSYA. Therefore, this research is expected to have applications for quick and accurate determination of HSYAand can also be used for various immunoassays with other antigens..
Keywords— Porous Silicon; Waveguide; Label-Free Biosensor; HydroxySafflor Yellow A.
I. INTRODUCTION
Hydroxysafflor yellow A (HSYA, MW: 432 Da) is the main chemical component of the Chinese herb Carthamus tinctorius L., which is extensively used in traditional Chinese medicine for treatment of cerebrovascular and cardiovascular diseases (Wei et al., 2005). The levels of HSYA content are the key criterion to evaluate the quality of Carthamus tinctorius L. and the corresponding traditional Chinese medicine. Thus, it is significant to explore a quick and accurate determination of such small molecules for judging the quality of medicines, monitoring the quality of pharmaceutical process and conducting pharmacokinetic analysis. Traditionally, the method for determination of HSYA is high-performance liquid chromatography (HPLC), a sensitive but cumbersome technique which requires expensive equipment. As an important analytical method, the immunoassay can be used as a tool for determining HSYA, however, traditional immunoassays such as enzymelinked immunosorbent assay (ELISA) involve labeling and a longer detection time.
An optical biosensor combined with immunoassay technology offers the advantage of being highly selective and sensitive while remaining simple and label-free. Porous silicon (PSi) is an ideal optical biosensor material because of its high surface area, low cost, wide availability (Meskini et al., 2007; Lin et al., 1997), and compatibility with standard IC processes. In previous work, we investigated single and multilayered PSi as a biosensor platform for determining the artificial immunogen of HSYA (Lü et al., 2009). Saarinen and coworkers (Rong et al., 2008; Saarinen et al., 2005) reported that using a PSi waveguide for detecting DNA fragments has many advantages over other thin resonant or PSi-based biosensors, such as high sensitivity and fast-response; however, the surface pore diameters of the PSi waveguide they reported are only about 20 nm, which is too small for easy detection of large size biomolecules such as the anti-HSYA antibodies.
To enable large size molecules to infiltrate the PSi waveguide easily thus allowing detection of HSYA, in this experiment, an immunoassay based on macro-PSi resonant waveguide (pore size > 60 nm), was developed, building on the published work of Rong et al. (2008) who described the nanoscale PSi waveguide. We have successfully used a crosslink method linker HSYA antibody to the macro-PSi waveguide and then measured the shift of the waveguide resonance angle before and after the antigen-antibody reaction. The results show that the shift of the waveguide resonance angle increases with HSYA concentration. Thus, the macro-PSi waveguide is highly sensitive, a finding which lays a foundation for the development of simple and label-free immunosensors.
II. METHODS
A. Principle
As shown in Fig. 1, the schematic of the macro-PSi waveguide builds on the published work of Rong et al. (2008) who describe the same PSi waveguide structure. Two layers of PSi were formed on the silicon substrate: a top layer of low porosity and high refractive index, and a bottom layer of high porosity and low refractive index. An air gap separated the PSi waveguide from the prism, which had a refractive index of 1.811. PSi played the same role as metal in a surface plasmon resonance (SPR sensor) (Saarinen et al., 2005). There is a resonance dip in the spectrum when the incidence angle is at the vicinity of the resonance angle (Rong et al., 2008). Before and after the antigenantibody reaction, the refractive index of the PSi waveguide changed, resulting in the shift of the waveguide resonance angle.

Fig. 1: Schematic of the PSi waveguide.
B. Experimental setup
In Figure 2 a wide view of our experiment set-up is shown (Liu et al., 2005). In our setup, a light waveguide parameter measurer (Liu et al., 2005) is used to measure the waveguide reflectance as a function of angle. The light waveguide parameter measurer enables highly sensitive measurement with an angular resolution of 0.000625°. The light source is a collimation laser emitting radiation at l=650 nm. There is an air layer between the waveguide and a ZF7 prism (n=1.811).

Fig.2: Experimental setup (Liu et al., 2005).
C. Macro-PSi waveguide formation
P-type <100> (0.01 Ωcm) silicon wafer is employed for the preparation of macro-PSi waveguide samples, anodized by a 50% (1:1 in volume) solution of HF (48 wt %) and ethanol (98%). The application of 10 mA/cm2 for 60 s forms the top PSi layer and 50mA/cm2 for 60 s forms the second PSi layer. The thickness of the top layer is approximately 520 nm (Fig. 3) and the second layer is only required to be thick enough to prevent leakage (Rong et al., 2008). After etching, PSi surface post-treatment is conducted using cathode reduction (Yu et al., 2003), a technique which boosts the stability, mechanical intensity, and thermal stability of PSi (Yu et al., 2003). To enlarge the pore diameter, each chip was exposed to 1 mM KOH solution for 5 min (DeLouise et al., 2005). Then, the samples were rinsed with deionized (DI) water and dried with nitrogen flow.

Fig. 3: Cross-sectional SEM of PSi waveguide.
D. Chemical Modification of PSi waveguide
The process of the chemical modification for biosensing has been described in detail elsewhere (DeLouise et al., 2005; Ouyang et al., 2004) and is reviewed here briefly. The Macro-PSi waveguide first must be oxidized as a prerequisite for functionalization prior to HSYA immobilization (Rong et al., 2008; Ouyang et al., 2004), using 30% H2O2 solution at 45°C for 2 h. After oxidation, the sample was derivatized with amine silanization followed by glutaraldehyde. Firstly, the chips were treated with a 5% solution of APTES (aminopropyltriethoxysilane) and a hydroalcoholic mixture of water and methanol (1:1) for 20 min at room temperature. Next, we washed the sample with DI water and dried in nitrogen stream. Then, the silanized chip was baked at 100°C for 10 min. After this, each sample was immersed in a 2.5% Glutaraldehyde solution for 20 minutes and then rinsed in PBST solution (Fig. 4).

Fig.4: Schematic of silanization and crosslinking of biomolecule into PSi waveguide.
E. Binding of antibody and detection of HSYA
HSYA is a small molecular hapten possessing immunoreactivity but not possessing immunogenicity. In order to elicit an immune response, HSYA must be combined with a large carrier such as BSA(bovine serum albumin). The artificial immunogen of HSYA, HSYA-BSA was prepared by the immediate coupling method, and the polyclonal antiserum was produced from two rabbits immunized with conjugates HSYA-BSA. The covalent bond of the HSYA antibodies on the PSi waveguide surface was based on a two step process (Fig. 4). We first dropped 30 µL HSYA antibodies solution onto the PSi waveguide to completely cover its surface area, and then incubated the system at 37. for 2 h. After this the chips were exposed to 3% OVA(ovalbumin) for 2 h to prevent nonspecific adsorption. Next, we dropped onto each sample 30 µL of different concentrations of target HSYA for 2 h at 37°C, followed by rinsing in PBST buffer.
III. RESULTS AND DISCUSSION
A. Pore characterization
P+ wafers usually produce pores of 5-20 nm in diameter, which is too small to admit large molecules such as antibodies. De Louise et al. (2005) have demonstrated that dilute KOH post-etching can successfully increase the pore size without destroying the PSi structure. Figure 5 shows a plan-view scanning electron microscope (SEM) image representative of the macro-PSi wave-guide samples used. Pores ranging from 60 to 120 nm in diameter are evident. HSYA is very small compared to BSA (MW of 432 Da compared to 67 kDa for BSA), while the spheroid dimensions of BSA are 4 nm by 14 nm (Peter, 1985). Thus, the HSYA antibodies produced by HSYA-BSA can easily infiltrate the macro-PSi waveguide.

Fig.5: Plan-view SEM of macro-PSi waveguide.
B. FTIR spectra of surface modification
The chemical reactions were monitored by Fourier transformed infrared spectroscopic microscopy (FTIR). Fourier-transformed infrared (FTIR) spectra were obtained in absorbance mode with a Brucker VERTEX70 spectrometer equipped with a Middle Infrared (MIR) source. All spectra were collected with 240 scans for the absorbance of the sample, with 4 cm-1 resolution. Figure 6 and Fig. 7 show the FTIR spectrum of the macro-PSi waveguide at various stages of immobilization between 500 and 4000 cm-1. In Fig. 6, the initial sample displayed Si-H bonds around 2064 cm-1 (Fernandez et al., 2008). After oxidation, a broad band appeared around 3500 cm-1 due to the Si-OH bonds on the surface (Fernandez et al., 2008; Li et al., 2002), and the sample also showed a band around 1007 cm-1 due to Si-O bond formation (Fernandez et al., 2008; Xu et al., 1997). In Fig. 7, the FTIR spectra of the PSi after the silanization process are shown: APTES characteristic peaks of the ethylic (at 1651, 1558 cm-1) and also the -CH (at 2927cm-1) groups are well evident (De Stefano et al., 2008). Finally, in Fig. 7, after the GA treatment, the characteristic imine-C=N bonds (at 1546 cm-1) due to the reaction with APTES are easily recognized (De Stefano et al., 2008; De Tommasi et al., 2008).

Fig. 6: FTIR spectra of macro-PSi waveguide: Before any treatment and after oxidation

Fig. 7: FTIR spectra of macro-PSi waveguide: after the APTES functionalization process and after Binding GA
C. Control experiment with negative serum
To verify that the waveguide resonance shift is due to selective antigen-antibody binding, and in order to further support the specificity of the method, a control experiment with negative serum instead of anti-HSYA antibodies (from the same rabbit was performed, also with the HSYA concentration10 µg/mL. Figure 8 shows that there is almost no shift of the internal angle and this immunosensor possesses high specificity.

Fig. 8: No resonance angle shift upon exposure of PSi waveguide to negative serum
D. Dose-response curves
The coupling angle shifts before and after the antigenantibody reaction as a function of the HSYA concentration. Figure 9 shows the dose-response curve as a function of the HSYA concentration. It shows a linear response between 0.01 and 1µgmL-1 with a correlation coefficient of 0.998. The sensitivity of 1.688degµg-1 m L-1 was calculated by estimating the slope. Because the angular resolution of the light waveguide parameter measurer is 0.000625°, the lowest detection limit of the macro-PSi waveguide immunosensor is 0.000625 deg/ 1.688  . However, when the HSYA concentration was greater than 1
. However, when the HSYA concentration was greater than 1 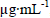 , the coupling angle shifts became much smaller, indicating that the surface of the PSi waveguide was saturated with HSYA. Compared to conventional ELISA, this immunoassay method for determining HSYA by using a macro-PSi waveguide is label-free and detection time is reduced. It is expected that this method has applications for quick and accurate component detection of Carthamus tinctorius L. Experiments are in progress to increase reversibility of the PSi waveguide sensor and enhance the detection limit of HSYA.
, the coupling angle shifts became much smaller, indicating that the surface of the PSi waveguide was saturated with HSYA. Compared to conventional ELISA, this immunoassay method for determining HSYA by using a macro-PSi waveguide is label-free and detection time is reduced. It is expected that this method has applications for quick and accurate component detection of Carthamus tinctorius L. Experiments are in progress to increase reversibility of the PSi waveguide sensor and enhance the detection limit of HSYA.

Fig. 9: Dose-response curve. Optical variation as a function of the HSYA concentration.
IV. CONCLUSIONS
We have experimentally demonstrated a new optical immunoassay method based on a macro-PSi waveguide as an immunosensor for detection of the antigenantibody reaction between HSYA and the specificity of the polyclonal anti-HSYA antibodies. HSYA antibodies were successfully immobilized to the immunosensor. After HSYA as immunogen was attached to the macro- PSi waveguide, the shift of the waveguide resonance angle increased with the increase of HSYA concentration with the detection limit of  . Consequently, this method lays a foundation for the practical application of determining HSYA quickly and effectively in therapeutic drug monitoring.
. Consequently, this method lays a foundation for the practical application of determining HSYA quickly and effectively in therapeutic drug monitoring.
ACKNOWLEDGMENTS
This work was supported by the financial support by the National Natural Science Foundation of China (No. 60968002) and Program for New Century Excellent Talents in University of the P.R. China (Grant NCET-05-0897)and by Scientific Research Project for Universities in Xinjiang (Grant XJEDU 2006I10).
REFERENCES
1. Louise, L.A., M.K. Peng and L. Benjamin, "Miller cross-correlation of optical microcavity biosensor response with immobilized enzyme activity insights into biosensor sensitivity," Anal.Chem, 77, 3222-3230 (2005). [ Links ]
2. De Tommasi, E., L. DeStefano, I. Rea, V. DiSarno, L. Rotiroti, P. Arcari, A. Lamberti, C. Sanges and I. Rendina., "Porous silicon based resonant mirrors for biochemical sensing," Sensors, 8, 6549-6556 (2008). [ Links ]
3. De Stefano, L., A. Lamberti, L. Rotiroti and M. De Stefano, "Interfacing the nanostructured biosilica microshells of the marine diatom Coscinodiscus wailesii with biological matter," Acta Biomaterialia, 4, 126-130 (2008). [ Links ]
4. Fernandez, R.E., E. Bhattacharya and A.Chadha, "Covalent immobilization of Pseudomonas cepacia lipase on semiconducting materials," Appl. Surf. Sci., 254, 4512-4519 (2008). [ Links ]
5. Li,H.L., A.P. Fu, S.S. Xu, G.L. Guo, L.L. Gui and Y.Q. Tang, "In situ silanization reaction on the surface of freshly prepared porous silicon," Langmuir, 18, 3198-3202 (2002). [ Links ]
6. Lin, V.S.-Y., K. Motesharei, K.-P.S. Dancil, M.J. Sailor and M.R. Ghadiri, "A porous silicon-based optical interferometric biosensor," Science, 278, 840-843 (1997). [ Links ]
7. Liu, X.B., Q.S. Shen, Z.Q. Cao, X.X. Deng and H.G. Li, Light waveguide parameter measurer, China Patent, ZL 200410089285.7 (2005). [ Links ]
8. Lü X.Y., T. Qinggele, M. Xiang Z.H. Jia, R. Li, F.R. Zhong, J.W. Li and F.C. Zhang, "Optical detection of immunoreaction based on the porous silicon Bragg reflector," Chem. J. Chinese Universities, 30, 2381- 2386 (2009). [ Links ]
9. Meskini, O., A. Abdelghani, A. Tlili, R. Mgaieth, N. Jaffrezic-Renault and C. Martelet, "Porous silicon as functionalized material for immunosensor application," Talanta, 71, 1430-1433 (2007). [ Links ]
10. Ouyang, H., A.L. De Louise, M. Christophersen, B.L. Miller and P.M. Fauchet, "Biosensing with one dimensional photonic bandgap structure," Proceedings of SPIE, 5511, 71-80 (2004). [ Links ]
11. Peter, T., "Serum albumin," Adv. Protein Chem., 37, 161-245 (1985). [ Links ]
12. Rong, G., A. Najmaie, J.E. Sipe and S.M. Weiss, "Nanoscale porous silicon waveguide for label-free DNA sensing," Biosens. Bioelectron., 23, 1572-1576 (2008). [ Links ]
13. Saarinen, J.J., S.M. Weiss, P.M. Fauchet and J.E. Sipe, "Optical sensor based on resonant porous silicon structures," Opt. Express, 13, 3754-3764 (2005). [ Links ]
14. Wei, X., H. Liu, X. Sun, F. Fu, X. Zhang, J. Wang, J. An and H. Ding, "Hydroxysafflor yellow A protects rat brains against ischemia-reperfusion injury by antioxidant action," Neuroscience Letters, 386, 58-62 (2005). [ Links ]
15. Xu, Z., Q. Liu and J.A. Finch, "Silanation and stability of 3-aminopropyl triethoxy silane on nanosized superparamagnetic particles: I. Direct silanation," Appl. Surf. Sci., 120, 269-278 (1997). [ Links ]
16. Yu, X.W., R. Zhu, Z. Zhu, T. Ying and A. Li, "Drying of porous silicon," Chinese Journal of Semiconductors, 6, 663-667 (2003). [ Links ]
Received: December 11, 2009.
Accepted: August 25, 2011.
Recommended by Subject Editor Ana Lea Cukierman.














