Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Latin American applied research
versión impresa ISSN 0327-0793
Lat. Am. appl. res. vol.42 no.3 Bahía Blanca jul. 2012
Fixed-bed column of surface modified activated carbons for adsorption of mercury (II) in aqueous solution
H.S. Silva, D.L. Granados and S.V. Ruiz
Instituto de Ingeniería Química - Facultad de Ingeniería - Universidad Nacional de San Juan - Argentina Av. Libertador San Martín 1109 (Oeste) - 5400 San Juan Argentina. hsilva@unsj.edu.ar
Abstract— Activated carbons are universal adsorbents that can be obtained from a wide variety of raw materials, and have been found to be very effective for mercury removal from water. This contribution presents a comparative adsorption in column study of activated carbons obtained by physical activation of carbonized eucalyptus wood with water vapour, as adsorbents of mercury in liquid phase. Two samples were treated with sulphuric acid and with carbon disulphide, in order to introduce sulphur as a heteroatom onto the adsorbent surface. Adsorption in column assays was performed and the breakthrough curves were used to compare their performance. The sulphurization treatments produce important variations on the textural properties of the solid, on the zero charge point, and on the number of acid and basic surface groups. From the analysis of these factors, the dependency of the adsorption capacity of a solid with the pH of the solution can be explained.
Keywords— Mercury; Adsorption in Column; Modified Activated Carbon.
I. INTRODUCTION
Mercury and mercury compounds are important and useful industrial materials which have been used for a long time as pigments in inks (cinnabar, red sulphide), as aids to early metallurgy (gilding copper) and instrumentation (thermometers, barometers). Many industries have resort to the use mercury or mercury compounds in their main process (i.e. recovery of gold from its ores, manufacture of chlorine and sodium hydroxide by electrolysis of brine, etc.), or are present in their wastewaters.
Mercury is one of the most toxic metals present in the environment. Once it enters the food chain, large concentrations of this element accumulate in humans and animals, causing numerous adverse effects on their health.
In order to prevent the problems due to the toxicity of mercury, several technologies have been proposed to remove this metal from aqueous media, which include ion exchange, adsorption, sulphide precipitation, electrodeposition, solvent extraction and membrane processes (Patterson, 1997). Among the available technological alternatives for the removal of trace metals from water, adsorption has been considered an economically feasible one (Antochshuk et al., 2003; Babic et al., 2002; Blázquez et al., 2009; Costa et al., 2005; Han et al., 2009; Huang et al., 2009; Inbaraj and Sulochana, 2006, Kadirvelu et al., 2001; Namasivayam and Kadirvelu, 1999; Velicu et al., 2007).
Activated carbons are universal adsorbents that can be obtained from a wide variety of raw materials including wastes of industrial activities (Amaya et al., 2007; Baçaoui et al., 2001; Budinova et al., 2008; Deiana et al., 2004; Namasivayam and Sangeetha, 2006). These porous solids have been found to be a very effective alternative for mercury removal from water (Ekinci et al., 2002; Goel et al., 2005; Kadirvelu et al., 2004; Lee and Park, 2003; Mullett et al., 2007; Ranganathan 2003; Yardim et al., 2003; Zenga et al., 2004).
Adsorption in liquid phase is a complex phenomenon because both, solute and solvent, compete for the solid surface. Briefly, the adsorption of a solute depends on its molecular size and chemical properties, and on the textural properties and surface chemistry of the adsorbent (Gregg and Sing, 1982; Maroto-Valera et al., 2005).
Most of the mercury in solution exists as complexed species, which can be either positive, negative or neutral, depending on the composition and pH of the solution. The situation is further complicated because the type of species preferentially adsorbed and the extent of adsorption may depend on the state of ionization of the surface which, in turn, is dependent on the solution pH (Carrott et al., 1998).
In some cases the pH of an effluent cannot be modified (for example, when great volumes of liquid have to be decontamined or when this modification produces an environmental problem), and the capture of a contaminant can be enhanced by surface modification of the adsorbent by means of suitable treatments (Domingo-García et al., 2000; Li, 2003; Pastor-Villegas et al., 2007; Perez-Cadenas et al., 2003; Rios et al., 2003). In other cases it is possible to change the pH of the solution in order to improve the adsorption.
In the past few years, considerable attention has been devoted to develop surface-modified adsorbents in order to enhance the adsorption capacity of solids to eliminate mercury from aqueous media. Sulphur has been reported as an element which favours the adsorption of mercury (Anoop and Anirudhan, 2002; Gómez Serrano et al., 1998; Hsi et al., 2002; Manchón-Vizuete et al., 2005; O'Dowd et al., 2004; Vitolo and Pini 1999), therefore, the surface treatments that incorporate sulphur to activated carbons should improve the entrapment of mercury derived species. Most of these works have been carried out as batch studies and it is necessary to examine any adsorbent in continuous operation before its potential for mercury adsorption could be properly assessed (Al-Degs et al., 2009; El Qada et al., 2006; Goyal et al., 2009; Ruey-Shin et al., 2003; Wang et al., 2003).
This contribution presents a comparative adsorption in column study of three activated carbons obtained from eucalyptus wood, as adsorbents of mercury in liquid phase. The first adsorbent was obtained by physical activation of carbonized eucalyptus wood with water vapour. Two samples of this activated carbon were separated and treated with sulphuric acid and with carbon disulphide, respectively, in order to introduce sulphur as a heteroatom onto the adsorbent surface.
Adsorption in column assays to entrap mercury were performed for all the activated carbons and the breakthrough curves were used to compare their performance.
The structural and textural parameters of the adsorbents, as well as the operation variables (pH and temperature) were analized and their influence on the adsorption capacity of the solids was discussed.
II. METHODS
A. Experimental
Preparation of Adsorbents
The adsorbents were obtained from eucalyptus wood (EW). The start material was carbonized in a 5 L stainless steel retort, electrically heated, in absence of air, from room temperature to 773 K and kept at the final temperature for 2 hours.
The carbonized material (CEW) was placed as a fixed bed in a 30 mm internal diameter stainless steel reactor, electrically heated, and activated with water vapour, following the protocol detailed in previous works (Deiana et al., 1998; Deiana et al., 2004). Basically, the reactor was heated from room temperture to the activation temperature (1153 K) under nitrogen atmosphere. Then, the nitrogen stream was replaced by a water vapour flow (1.7 g of steam per g of carbonized material and per hour). An activation time of 105 minutes was adopted. When the activation process finished, the whole system was cooled from 1153 K to room temperature under nitrogen flow.
Two fractions of activated material (AC) were used to carry out the surface treatments of the adsorbent in order to incorporate sulphur as heteroatom onto the carbon surface. In one of the surface treatments, 50 g of AC were soaked in 250 mL of concentrated sulphuric acid, at room temperature, under stirring, for 24 hours. The liquid fraction was separated by filtration and the solid was washed free of acid with water and dried at 383 K for 3 hours. The solid was ground and sieved to -40+60 mesh particle size and labeled as AC-H2SO4.
The second sulphurization treatment was carried out using carbon disulphide as source of sulphur. 50 g of AC were soaked in 250 mL of carbon disulphide, at room temperature, and maintained under stirring for 24 hours. The sulphurized solid was filtered and dried in oven at 383 K for 3 hours, then was ground and sieved to -40+60 mesh particle size and labeled as AC-CS2.
Characterization of adsorbents
Textural properties
The specific surface and pore size distribution of AC, AC-H2SO4 and AC-CS2 were determined from the nitrogen adsorption isotherms at 77 K, carried out in a Quantachrome Nova 2200 sorptometer. The adsorption results were modelled by BET in order to determine specific surface, by DR to evaluate micropore volume and by BJH for pore size distribution.
Surface Chemistry
Acid and Basic Groups: Surface acid groups were determined by contacting 0.2 g of the solid with 20 mL of 0.1 M NaOH solution. The excess of NaOH was measured by titration with 0.1 M HCl solution. Surface basic groups were determined by contacting 0.2 g of the solid with 20 mL of 0.1 M HCl solution and the excess of HCl, by titration with 0.1 M NaOH solution.
Point of zero charge: The pH of the point of zero charge, i.e. the pH above which the total surface of the carbon particles is negatively charged, was measured by the so-called mass titration method proposed by Noh and Schwartz (1989). Briefly, three aqueous solutions of different initial pH were prepared from a 0.01 M NaNO3, using 0.01 M NaOH and 0,01 M HNO3 solutions for its regulation in order to set the desired initial pH value. Six vials containing different amounts of the adsorbent under study were filled with 20 ml of solutions at different initial pH. The equilibrium pH was measured after four days. The three curves at different initial pH, converge at a single point (pHPZC).
Adsorption in Column Studies
The experimental arrangement for the adsorption in column studies is shown in Fig. 1. Each adsorbent was placed in a 9 mm internal diameter column as a packed bed. The columns were charged with 2.5 g of each dried adsorbent (-40 + 60 mesh particle size) to give a total column height of 12 cm. A 1 cm-thick layer of alumina wool at the top and at the bottom was used to ensure a closely packed bed. The columns were operated in a downflow configuration at an hydraulic loading of 1.81 L/(m2 h). Feed was prepared in a 10 L capacity tank at an initial HgCl2 concentration of 40 mg/L (C0) and was continuously pumped to the column.

Figure 1. Scheme of the experimental arrangement for the adsorption in column tests. 1 Alumina wool layer. 2 bed of adsorbent. 3 Heat exchange jacket. 4 Peristaltic pump. 5 Influent solution tank. 6 Effluent solution tank. 7 Inlet temperature stabilitazion stream. 8 Outlet temperature stabilitazion stream.
The effluent was sampled at different time intervals. The adsorption tests were performed at pH = 3.0; pH = 5.8 and pH = 12.0 to study the pH influence. The pH was adjusted using 0.1 M HCl and 0,1 M NaOH solutions. Adsorption tests at 293 K and 313 K were carried out to study the temperature influence. The temperature of the stabilization stream and the solution tank were adjusted using a Colora Ultra Thermostat NB 36130.
The concentration of residual mercury in the solution was determined by spectrophotometry UV-VIS in a Hach DR 2010 model equipment. The liquid sample containing the non-adsorbed mercury was complexed with potasium iodide and rhodamine, according to the protocol detailed by Muralidhara (1998) and Kardivelu et al. (2004). The concentration of mercury in solution was determined measuring the absorbance at 575 nm.
B. Results and discussion
Textural Characterization of the Adsorbents
Figures 2 and 3 show the adsorption isotherms and the pore size distributions corresponding to the three studied adsorbents.

Figure 2. Adsorption desorption isotherms of nitrogen at 77K.

Figure 3. Pore size distribution modelled by BJH.
Table 1 summarizes the main textural parameters of the adsorbents. The adsorption isotherms corresponding to AC-H2SO4 and AC-CS2 show important differences compared with that of AC, mainly in the position of the knee of the isotherm, which basically indicates that the microporous structure has been substantially modified by the sulphurization treatments
Table 1. Textural properties of the adsorbents
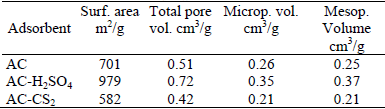
The increase of the micropore volume in the AC-H2SO4 adsorbent could be the result of a chemical activation caused by the sulphuric acid which generates more cavities.
The decrease of the micropore volume observed for the AC-CS2 sample could be associated to a pore blocking effect due to the surface sulphurized groups. An increment in the mesoporous volume corresponding to the AC-H2SO4 adsorbent is also produced. However, from the hysteresis loop shape, the mean connectivity of the porous net, does not vary after the sulphurization treatments (Seaton, 1991; Liu and Seaton 1992; Liu and Seaton 1994).
The adsorption capacity of a solid depends, among other properties, on specific surface area, the pore size distribution and the mean connectivity of the porous net. Because of its higher specific surface and pore volume AC-H2SO4 should be a better adsorbent than AC and AC-CS2. This also applies from the surface chemistry point of view, considering that in AC-H2SO4 the surface sulphurized groups are specially favourable for mercury adsorption (Anoop and Anirudhan, 2002; Gómez Serrano et al., 1998; Hsi et al., 2002; Manchón-Vizuete et al., 2005; O'Dowd et al., 2004; Vitolo and Pini, 1999).
A lower mercury adsorption capacity should be expected for the AC-CS2 adsorbent due to the significant decrease of specific surface and pore volume compared to the AC adsorbent. However the adsorption capacity of AC-CS2 adsorbent is higher than that of AC and AC-H2SO4 adsorbents, because its unfavourable textural properties are balanced by the surface chemistry which improves the mercury adsorption.
Surface Chemistry
The results of the surface chemistry characterization are summarized in Table 2. Important variations in the pHPZC value and in the acid groups/basic groups ratio have been produced after the sulphurization treatments and a different behavior of the adsorbents for the mercury adsorption should be expected.
Table 2. Surface acid and basic groups

Adsorption in Column Studies
The breakthrough curves corresponding to AC, AC-H2SO4 y AC-CS2 adsorbents at pH=5.8, are shown in Fig. 4. From the curves, it is evident a different behavior of the adsorbents for mercury entrapment. Although the AC-H2SO4 solid presents higher specific surface, micropore volume and macropore volume respect to the AC adsorbent (Table 1), mercury concentration starts raising at a slightly higher cumulative volume of effluent respect to the AC adsorbent (6 L approximately).

Figure 4. The breakthrough curves at pH= 5.8.
The breakthrough curve correponding to AC-H2SO4 adsorbent is markedly different to that of the AC solid which indicates that the height of mass transfer zone HMTZ (where adsorption in column occurs), is smaller than that of the AC solid. However, from the column efficiency for the mercury entrapment, there is no substancial difference between both adsorbents (the United States Environmental Protection Agency has been established 2 ppb for the maximum contaminant level for inorganic mercury in drinking water)
A substantially different performance shows the AC-CS2 adsorbent. Although it presents lower specific surface, micropore volume and mesopore volume compared with AC and AC-H2SO4, more than twice the cumulative volume of effluent, is necessary before mercury can be detected in the effluent stream.
This behavior can be due to the changes in the surface chemistry after the sulphurization treatments.
- pH influence
The adsorption capacity of the solids for mercury strongly depends on the pH of the solution.
Adsorption in liquid phase is a complex phenomenon. The molecules of the solute and of the solvent compete for the adsorption sites. The entrapment of a solute or a solvent onto the solid surface is the result of the forces between the chemical species in solution and the surface chemical groups of the solid. These forces have been studied theoretically from a long time but, until now, it is not possible to calculate the course of an adsorption test from known and independently determined parameters of the liquid and the solid molecules.
One of the features which influences the adsorption of a solute is the charge of the chemical specie in which the contaminant exists in solution (which depend of the pH and pCl of the solution), compared to the net charge of the solid surface (which depends of the pH of the solution). It is expected more adsorption rates when those charges are opposite.
The results of the adsorption tests at pH=3.0, pH=5.8 and pH=12 are shown in Fig. 5, Fig. 6 and Fig. 7, for the AC, AC-H2SO4 and AC-CS2 adsorbents, respectively. The adsorption capacity at pH=3 and pH=12 is substantially lower compared to that at pH=5.8.

Figure 5. Breakthrough curves for the AC adsorbent at different pH.

Figure 6. Breakthrough curves for the AC-H2SO4 adsorbent at different pH.

Figure 7. Breakthrough curves for the AC-CS2 adsorbent at different pH.
This behavior can be explained from the net surface charge of each adsorption test and the charge of the species in which mercury is in solution. For example, pHPZC= 8.56 for the AC-CS2 adsorbent. When the adsorption test is carried out at pH= 12, the net charge of the solid surface is negative.
At pCl= 3.8 and pH= 12 the dominant species in solution are neutral [Hg(OH)2]; [HgClOH] and negative [Hg(OH)2Cl-]. Then there will be electrostatic repulsion forces between the solid surface and the negative charged species influencing then negatively on the Hg cation adsorption. When the adsorption test is carried out at pH=5.8, the net charge of the solid surface is positive and the main species in solution is [Hg(OH)2]. In this case, no matter the surface net charge, there is not an important electrostatic effect compared to that at pH= 12 and the adsorbent capacity of the solid is higher. For the adsorption test at pH= 3, the net charge of the solid surface is positive and the main species in solution are [Hg++]; [HgCl+]; [HgOH+]. In this case there is a repulsion electrostatic effect between the solid surface and the positive charged species. Then, the adsorption of the mercury species is more difficult compared to that observed at pH= 5.8. A similar analysis for the AC and AC-H2SO4 adsorbents can explain the adsorption capacities observed at different solution pH.
- Temperature influence
The temperature of the adsorption tests does not influence the adsorption capacity of the studied adsorbents. As an example, Fig. 8 corresponding to the AC adsorbent, is included. Similar behavior is observed for the other tested adsorbents.

Figure 8. Breakthrough curves for the AC adsorbent at different temperature.
This is because although the adsorption process is exothermic in nature, the condensation of a solute onto the adsorbent surface only occurs by the displacement of a solvent molecule previously adsorbed (endothermic process). If the heat of adsorption of a solute molecule is similar to that of the desorption of the solvent molecule, the whole process will not be influenced by the temperature.
III. CONCLUSIONS
The activated carbon from carbonized eucalyptus wood is an adequate adsorbent for the entrapment of mercury from aqueous solutions and its adsorbent efficiency can be substantially enhanced by the modification of its surface chemistry. The improvement in the adsorbent capacity depends not only on the surface modifications but also on the speciation of the solute in the solution. Both factors depend on the pH of the solution.
The surface sulphurization treatments produce important variations on the zero charge point and on the textural properties of the solid (basically specific surface and pore size distribution) and on the number of acid and basic surface groups.
From the analysis of the above mentioned factors, the dependency of the adsorption capacity of a solid with the pH of the solution can be explained. The temperature does not influence significantly the adsorption properties of the solid studied.
ACKNOWLEDGMENT
The authors wish to acknowledge the financial support provided by the Universidad Nacional de San Juan, Argentina.
REFERENCES
1. Al-Degs, Y.S., M.A.M. Khraisheh, S.J. Allen and M.N. Ahmad, "Adsorption characteristics of reactive dyes in columns of activated carbon," J. Hazard. Mater., 165, 944-949 (2009). [ Links ]
2. Amaya, A., N. Medero, N. Tancredi, H. Silva and C. Deiana, "Activated carbon briquettes from biomass materials," Bioresour. Technol., 98, 1635-1641 (2007). [ Links ]
3. Anoop, K. and T. Anirudhan, "Removal of mercury (II) from aqueous solutions and chlor-alkali industry effluent by steam activated and sulphurised activated carbons prepared from bagasse pith: kinetics and equilibrium studies," J. Hazard. Mater.B ,92, 161-183 (2002). [ Links ]
4. Antochshuk, V., O. Olkhovyk, M. Jaroniec, I. Park and R. Ryoo, "Benzoylthiourea-modified mesoporous silica for mercury (II) removal," Langmuir, 19, 3031-3034 (2003). [ Links ]
5. Babic, B., S. Milonjic, M. Polovina. S. Cupic and B. Kaludjerovic, "Adsorption of zinc, cadmium and mercury ions from aqueous solutions on an activated carbon cloth," Carbon, 40, 1109-1115 (2002). [ Links ]
6. Baçaoui, A., A. Yaacoubi, A. Dahbi, C. Bennouna, R. Phan Tan Luu, F.J. Maldonado-Hodarc, J. Rivera-Utrillac and C. Moreno-Castilla, "Optimization of conditions for the preparation of activated carbons from olive-waste cakes," Carbon, 39, 425-432 (2001). [ Links ]
7. Blázquez, G., F. Hernáinz, M. Calero, M.A. Martín-Lara and G. Tenorio, "The effect of pH on the biosorption of Cr(III) and Cr(VI) with olive stone," Chem. Eng. J., 148, 473-479 (2009). [ Links ]
8. Budinova, T., N. Petrova, J. Parrab and V. Baloutzov, "Use of an activated carbon from antibiotic waste for the removal of Hg(II) from aqueous solution," J. Environ. Manage., 88, 165-172 (2008). [ Links ]
9. Carrott, P., M. Ribeiro Carrott and J. Nabais, "Influence of surface ionization on the adsorption of aqueous mercury chlorocomplexes by activated carbons," Carbon, 36, 1-17 (1998). [ Links ]
10. Costa, L., M. Araujo, K. Sapag, M. Sardella, H. Silva, C. Deiana and M.R. Lago, "High surface area functionalized carbon briquettes: A novel adsorbent for metals from water," J. Braz. Chem. Soc., 16, 899-902 (2005). [ Links ]
11. Deiana, C., L. Petkovic and S. Noriega, "Carbón activado a partir de materias primas regionales," Inf. Tecnol., 9, 89-93 (1998). [ Links ]
12. Deiana, A.C., D.L. Granados, L.M. Petkovic, M.F. Sardella and H.S. Silva, "Use of grape must as a binder to obtain activated carbon briquettes," Braz. J. Chem. Eng., 21, 585-591 (2004). [ Links ]
13. Domingo-García, M., F. López-Garzón and M. Pérez-Mendoza, "Effect of some oxidation treatments on the textural characteristics and surface chemical nature of an activated carbon," J. Colloid Interface Sci., 222, 233-240 (2000). [ Links ]
14. Ekinci, E., T. Budinova, F. Yardim, N. Petrov, M. Razvigorova and V. Minkova, "Removal of mercury ion from aqueous solution by activated carbons obtained from biomass and coals," Fuel Process. Technology., 77-78, 437- 443 (2002). [ Links ]
15. El Qada, E.N., S.J. Allen and G.M. Walker, "Adsorption of basic dyes onto activated carbon using microcolumns," Ind. Eng. Chem. Res., 45, 6044-6049 (2006). [ Links ]
16. Goel, J., K. Kadirvelu, C. Rajagopal and V. Garg, "Removal of mercury (II) from aqueous solution by adsorption on carbon aerogel: Response surface methodological approach," Carbon, 43, 195-213. (2005). [ Links ]
17. Gómez Serrano, V., A. MacíasGarcía, A. Espinosa Mansilla and C. Valenzuela Calahorro, "Adsorption of mercury, cadmiun and lead from aqueous solution on heat-treated and sulphurized activated carbon," Water Res., 32, 1-4 (1998). [ Links ]
18. Goyal, M., M. Bhagat and R. Dhawan, "Removal of mercury from water by fixed bed activated carbon columns," J. Hazard. Mater., 171, 1009-1015 (2009). [ Links ]
19. Gregg, S. and K. Sing, Adsorption, Surface Area and Porosity, 2nd ed., Academic Press, London (1982). [ Links ]
20. Han, R.P., J.J. Zhang, P. Han, Y.F. Wang, Z.H. Zhao and M.S. Tang, "Study of equilibrium, kinetic and thermodynamic parameters about methylene blue-adsorption onto natural zeolite," Chem. Eng. J., 145, 496-504 (2009). [ Links ]
21. Hsi, H., M. Rood, M. Rostam-Abadi, S. Chen and R. Chang, "Adsorption properties of sulfur-impregnated adsorbents," J. Environ. Eng., 128, 1080-1089 (2002). [ Links ]
22. Huang, G.L., J.X. Shi and T.A.G. Langrish, "Removal of Cr(VI) from aqueous solution using activated carbon modified with nitric acid," Chem. Eng. J., 152, 434-439 (2009). [ Links ]
23. Inbaraj, S. and N. Sulochana, "Mercury adsorption on a carbon sorbent derived from fruit shell of Terminaliacatappa B.," J. Hazard. Mater. B., 133, 283-290 (2006). [ Links ]
24. Kadirvelu, K., K. Thamaraiselvi and C. Namasivayam, "Removal of heavy metals from industrial wastewaters by adsorption onto activated carbon prepared from an agricultural solid waste," Bioresour. Technol., 76, 63-65 (2001). [ Links ]
25. Kadirvelu, K., M. Kavipriya, C. Karthika, N. Vennilamani and S. Pattabhi, "Mercury (II) adsorption by activated carbon made from sago waste," Carbon, 42, 745-752 (2004). [ Links ]
26. Lee, S. and Y. Park, "Gas-phase mercury removal by carbon-based sorbents," Fuel Process. Technology, 84, 197- 206 (2003). [ Links ]
27. Li, Y., "Importance of activated carbon's oxygen surface functional groups on elemental mercury adsorption," Fuel, 82, 451-457 (2003). [ Links ]
28. Liu, H. and N. Seaton, "Determination of the connectivity of porous solids from nitrogen sorption measurements-II. Generalisation," Chem. Eng. Sci., 47, 4393-4404 (1992). [ Links ]
29. Liu, H. and N. Seaton, "Determination of the connectivity of porous solids from nitrogen sorption measurements-III. Solids containing large mesopo-rous," Chem. Eng. Sci., 49, 1869-1878 (1994). [ Links ]
30. Manchón-Vizuete, E., A. Macías-García, A. NadalGisbert, C. Fernández-González and V. Gómez-Serrano, "Adsorption of mercury by carbonaceous adsorbents prepared from rubber of tyre wastes," J. Hazard. Mater. B, 119, 231-238 (2005). [ Links ]
31. Maroto-Valera, M., Y. Zhanga, E. Graniteb, Z. Tanga and H. Pennlineb, "Effect of porous structure and surface functionality on the mercury capacity of a fly ash carbon and its activated sample," Fuel, 84, 105-108 (2005). [ Links ]
32. Mullett, M., J. Tardio, S. Bhargava and C. Dobbsc, "Removal of mercury from an alumina refinery aqueous stream," J. Hazard. Mater., 144, 274-282 (2007). [ Links ]
33. Muralidhara, B, "Indirect complexometric determination of mercury using potassium iodide as selective masking agent," Turk J. Chem., 22, 215-219 (1998). [ Links ]
34. Namasivayam, C. and K. Kadirvelu, "Uptake of mercury(II) from wastewater by activated carbon from an unwanted agricultural waste by-product: coirpith," Carbon, 37, 79-84 (1999). [ Links ]
35. Namasivayam, C. and D. Sangeetha, "Recycling of agricultural solid waste, coir pith: Removal of anions, heavy metals, organics and dyes from water by adsorption onto ZnCl2 activated coir pith carbon," J. Hazard. Mater., 135, 449-452 (2006). [ Links ]
36. Noh, J. and J. Schwartz, "Estimation of the point of zero charge of simple oxides by mass titration," J. Colloid Interface Sci., 130, 157-164 (1989). [ Links ]
37. O'Dowd, W., R. Hargis, E. Granite and H. Pennline, "Recent advances in mercury removal technology at the National Energy Technology Laboratory," Fuel Process. Technol., 85, 533- 548 (2004). [ Links ]
38. Pastor-Villegas, J., J.M. Meneses-Rodrıíguez, J.F. Pastor-Valle and M. García-García, "Changes in commercial wood charcoals by thermal treatments," J. Anal. Appl. Pyrolysis, 80, 507-514 (2007). [ Links ]
39. Patterson, J., Capsule Report: Aqueous Mercury Treatment. National Risk Management Research Laboratory. Office of Research and Development. United States Environmental Protection (1997). [ Links ]
40. Perez-Cadenas, A., F. Maldonado-Hodar and C. Moreno-Castilla, "On the nature of surface acid sites of chlorinated activated carbons," Carbon, 41, 473-478 (2003). [ Links ]
41. Ranganathan, K., "Adsorption of Hg(II) ions from aqueous chloride solutions using powdered activated carbons," Carbon, 41, 1087-1092 (2003). [ Links ]
42. Rios, R., D. Alves, I. Dalmázio, S. Bento, C. Donnici and R. Lago, "Tailoring activated carbon by surface chemical modification with O, S, and N containing molecules," Mat. Res., 6, 129-135 (2003). [ Links ]
43. Ruey-Shin, J., L. Su-Hsia and W. Tsung-Yuan, "Removal of metal ions from the complexed solutions in fixed bed using a strong-acid ion exchange resin," Chemosphere, 53, 1221-1228 (2003). [ Links ]
44. Seaton, N., "Determination of the connectivity of porous solids from nitrogen sorption measurements," Chem. Eng. Sci., 46, 1895-1909 (1991). [ Links ]
45. Velicu, M., H. Fua, R.P.S. Suri and K. Woods, "Use of adsorption process to remove organic mercury thimerosal from industrial process wastewater," J. Hazard.Mater., 148, 599-605 (2007). [ Links ]
46. Vitolo, S. and R. Pini, "Deposition of sulfur from H2S on porous adsorbents and effect on their mercury adsorption capacity," Geothermics, 28, 341-354 (1999). [ Links ]
47. Wang, Y., L. Su-Hsia and J. Ruey-Shin, "Removal of heavy metal ions from aqueous solutions using various low-cost adsorbents," J. Hazard. Mater. B, 102, 291-302 (2003). [ Links ]
48. Yardim, M., T. Budinova, E. Ekinci, N. Petrov, M. Razvigorova and V. Minkova, "Removal of mercury (II) from aqueous solution by activated carbon obtained from furfural," Chemosphere, 52, 835-841 (2003). [ Links ]
49. Zenga, H., F. Jin and J. Guo, "Removal of elemental mercury from coal combustion flue gas by chloride-impregnated activated carbon," Fuel, 83, 143-146 (2004) [ Links ]
Received: February 8, 2011.
Accepted: September 22, 2011.
Recommended by subject editor: Pedro de Alcântara Pessôa














