Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Latin American applied research
versión impresa ISSN 0327-0793
Lat. Am. appl. res. vol.42 no.3 Bahía Blanca jul. 2012
Microwave-assisted extraction of phenolic and flavonoid compounds from Eucalyptus camaldulensis Dehn leaves as compared with ultrasound-assisted extraction
M. Gharekhani†, M. Ghorbani† and N. Rasoulnejad‡
† Department of Food Science & Technology, Gorgan University of Agricultural Sciences and Natural Resources, Beheshti Ave., Gorgan, 49138-15739, Iran. M.gharekhani@yahoo.com; Moghorbani@yahoo.com
‡ Department of Natural Resources, Isfahan University of Technology, Isfahan, Iran. N.rasoulnejad@yahoo.com
Abstract — In the present study, a microwave-assisted extraction (MAE) technique has been developed for extraction of phenolic and flavonoid compounds from eucalyptus leaves. Various experimental conditions, such as solvent type, ethanol concentration, MAE time, liquid/solid ratio and pre-leaching time before MAE were examined to optimize the extraction. Among the solvents tested, 50% aqueous ethanol extracted the highest phenolic and flavonoid contents from eucalyptus leaves. The extraction of phenolic and flavonoid compounds with MAE for 5 min was equivalent with ultrasound-assisted extraction (UAE) (60 min) and traditional extraction (24 h) methods. Time of extraction at room temperature and UAE was about 288 and 12 times higher than the needed with MAE, respectively. Due to the considerable saving in time, MAE was more effective than the traditional and UAE methods.
Keywords — Microwave-Assisted Extraction; Eucalyptus Leaves; Total Phenolic Compounds; Total Flavonoid Compound.
I. INTRODUCTION
Antioxidants are compounds that inhibit or delay the oxidation of other molecules by inhibiting the initiation or propagation steps in the oxidization chain reactions. Antioxidants are either natural or synthetic. Synthetic antioxidants have phenolic structures of various degrees of alkyl substitution, whereas natural antioxidants can be phenolic compounds (tocopherols, flavonoids, and phenolic acids), nitrogen compounds (alkaloids, chlorophyll derivatives, amino acids, and amines) or carotenoids as well as ascorbic acid (Larson, 1988; Hudson, 1990). Synthetic antioxidants such as butylated hydroxyl anisole (BHA) and butylated hydroxyl toluene (BHT) have been used as antioxidants since the beginning of the 20th century. Restrictions on the use of these compounds, however, are being imposed because of their carcinogenicity (Hwang et al., 2001). Thus, natural antioxidants have attracted much attention in recent years.
Crude extracts of fruits, herbs, vegetables, cereals, and other plant materials rich in phenolics are being used increasingly in the food industry because they retard oxidative degradation of lipids and thereby improve the quality and nutritional value of food. The importance of the antioxidant constituents of plant materials in the maintenance of health and protection from coronary heart disease and cancer is also raising interest among scientists, food manufacturers and consumers as the trend of future is moving toward functional food with specific health effects (Loliger, 1991). Flavonoids and other phenolics have been suggested to play a preventive role in the development of cancer and heart disease. The antioxidant activity of phenolics is mainly due to their redox properties, which allow them to act as reducing agents, hydrogen donators, and singlet oxygen quenchers. In addition, they have a metal chelation potential (Rice-Evans et al., 1995). Flavonoids and other plant phenolics, such as phenolic acids, stilbenes, tannins, lignans, and lignin, are especially common in leaves, flowering tissues and woody parts such as stems and barks (Larson, 1998). They are important in the plant for normal growth development and defense against infection and injury.
Among various aromatic plants, Eucalyptus (Family Myrtaceae) represented by over 700 species distributed throughout the world (Brooker and Kleinig., 2006; Batish et al., 2008) is one of the most-extensively planted pulpwood species. It consists of tall, magnificent and evergreen trees with fragrant foliage rich in oil glands and it is an excellent source of commercially important eucalyptus oil that finds extensive use in pharmaceutical, perfumery and industry (Batish et al., 2008). El-Ghorab et al. (2003) reported on "the promising antioxidative activities of ethanol extract from the leaves of Eucalyptus camaldulensis harvested from Egypt which contained gallic and ellagic acid as the major components".
The traditional extraction techniques of plant materials are mostly based on the correct choice of solvents and the use of heat and/or agitation to increase the solubility of the desired compounds and to improve the mass transfer. These techniques usually require longer extraction time thus running a severe risk of thermal degradation for most of the phyto-constituents (Mandal et al., 2007). Keeping in pace with such requirements recent times has witnessed the use and growth of new extraction techniques with shortened extraction time, reduced solvent consumption, increased pollution prevention concern and with special care for thermolabile constituents. Novel extraction methods including microwave assisted extraction (MAE), supercritical fluid extraction (SCFE), pressurized solvent extraction (PSE) have drawn significant research attention in the last decade. Some applications of MAE for biologically active compounds have appeared in the literature, such as extraction of essential oil from the fresh stems and leaves of Lippia alba (Stashenko et al., 2004), rosemary and peppermint (Chen and Spiro, 1994), extraction of embelin from dried berries of Embelia ribes (Latha, 2007), extraction of taxanes from Taxus biomass (Mattina et al., 1997), extraction of sanguinarine and chelerythrine from dry fruits of Macleaya cordata (Zhang et al., 2005), extraction of geniposidic acid and chlorogenic acid from dried bark of Eucommia ulmodies (Li et al., 2004), extraction of solanesol from tobacco leaves (Zhou and Liu., 2006), extraction of glycyrrhizic acid from licorice root (Pan et al., 2000) and extraction of polyphenols and caffeine from green tea leaves (Pan et al., 2003). The purpose of the present research was to develop a MAE method and compare it with UAE and traditional extraction methods for extraction of total phenolic and flavonoid compounds from eucalyptus leaves.
II. MATERIALS AND METHODS
A. Materials
Leaves of eucalyptus were collected in May 2008, in Naharkhoran region in Gorgan, Iran. Then, leaves were dried at room temperature under ventilated condition and ground to a fine powder, passed through a mesh number 60 sieve and were kept in an air- tight container at 4 °C until use. Gallic acid, quercetin and Folin-Ciocalteu were purchased from Merck Company.
B. Microwave-assisted extraction
A household microwave oven (Samsung, Korea) was modified in our laboratory by mounting of a magnetic stirrer, water condenser, temperature measurement and time controlling (Fig. 1). Dried eucalyptus leaves were mixed with an appropriate solvent. With power set on 600w, the suspension was irradiated under microwaves in pre-setting procedures (10 s power on, 15 s power off for three times to the desired temperature (about 75-85 °C) and then 3 s power on for heating and 10 s power off for cooling), and so on to the pre-set extraction time. Care was taken to prevent super boiling of the suspension. After the extraction, the flask was removed from the microwave oven and the extract was filtered through whatman No. 1 filter paper, and the resultant solution was collected in a volumetric flask. Determination of the total phenolic contents and the total flavonoid contents were carried out on the extracts immediately after the extraction step.
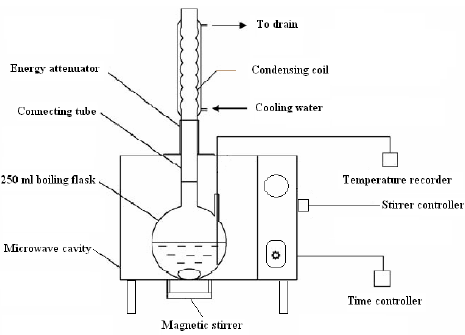
Fig. 1. The apparatus for microwave-assisted extraction
Experiments were carried out to determine the effect of type of solvents (water, methanol, ethanol, acetone, 50% aqueous methanol, 50% aqueous ethanol and 50% aqueous acetone), ethanol concentration (10, 20, 30, 40, 50, 60, 70, 80, 90 and 100% aqueous ethanol), MAE time (0.5, 1,2,3,5,7,9,11,13 and 15 min), liquid/solid ratio (10:1, 12.5:1, 15:1, 17.5:1, 20:1, 22.5:1 and 25:1 ml/g) and pre-leaching time before MAE (0, 20, 40, 60, 80, 100 and 120 min) on extraction efficiency of phenolic and flavonoid compounds
C. Ultrasound-assisted extraction
Ultrasound-assisted extraction was performed in an ultrasonic bath (Tecno3, Tecno-Gas.S.P.A, Italy). Samples were placed into a volumetric flask (250 ml), made up to volume with the extracting solvent and sonicated for different times at 30-40 °C temperature. After the extraction, the contents were filtered and samples were taken to determine of the total phenolic and flavonoid contents.
D. Determination of total phenolic contents
Total phenols were determined by Folin-Ciocalteu reagent (McDonald et al., 2001). A diluted extract of each plant extract (0.5 ml) or gallic acid (standard phenolic compound) was mixed with Folin-Ciocalteu reagent (5 ml, 1:10 diluted with distilled water) and aqueous Na2CO3 (4 ml, 1 M). The mixtures were allowed to stand for 15 min and the total phenols were determined by colorimetry at 765 nm. Total phenol values are expressed in terms of gallic acid equivalent (mg GA eq/g sample), which is a common reference compound.
E. Determination of total flavonoid contents
Aluminum chloride colorimetric method was used for flavonoids determination (Chang et al., 2002). Each plant extract (0.5 ml) was separately mixed with 1.5 ml methanol, 0.1 ml 10% aluminum chloride, 0.1 ml 1 M potassium acetate and 2.8 ml of distilled water. It was kept at room temperature for 30 min; the absorbance of the reaction mixture was measured at 415 nm with a double beam PG instruments UV/Visible spectrophotometer (USA). The calibration curve was prepared using quercetin standard solutions of 12.5 to 100 mg L-1 in methanol. Total flavonoid values are expressed in terms of quercetin equivalent (mg Qu eq/g sample), which is a common reference compound.
All experiments were run in triplicate. The deviation from the mean at the 95% confidence level was employed to determine the differences in results.
III. RESULTS AND DISCUSSION
A. The total phenolic content and total flavonoid content of eucalyptus leaves extracted with various solvents
Figure (2a) shows that water can extract highest contents of phenolic compounds. Ethanol, methanol and acetone were the next in the ranking. By adding water to these three solvents at equal proportion, the ethanol/water (1:1 v/v) solution gave higher concentration of phenolic compounds than the other solvent mixtures tested. The results also showed that addition of water to solvents gave higher efficiency of phenolic compounds compared to pure water. In Fig (2b), in contrast, it is shown that flavonoid compounds extraction decreased when water was added to solvents. The highest concentration of flavonoid compounds was extracted in methanol and ethanol. As the ethanol is less-toxic, can be mixed with water at different ratios and it is easily recovered by reduced pressure distillation, therefore, ethanol was used as the extraction solvent in the following study.


Fig. 2. The effect of various solvents on the extraction of total phenolic contents (a) and total flavonoid contents (b). Solvent: 100 ml; dried eucalyptus leaves: 5g; MAE for 9 min; liquid/solid ratio: 20:1 ml/g
B. Effect of ethanol concentration on extraction of total phenolic and total flavonoid compounds
Ethanol-water mixtures were used as the extraction solvent in the study. The effects of ethanol concentration in the extraction solvent (water) on the content of phenolics and flavonoids in eucalyptus leaves extracts are shown in Fig. 3. When ethanol concentration increased from 10% to 50% (v/v), the total phenolic content of the extracts increased from 54.3 to 84.8 mg GA eq./g sample. When ethanol concentration increased to 100% (v/v), the total phenolic content decreased sharply and at a concentration of 100 % (v/v), the total phenolic content was 49.4 mg GA eq./g sample. When pure ethanol was used to extract eucalyptus leaves, some lipid compounds might also be extracted, which limited the extraction of phenolics from eucalyptus leaves. A significant increase of the flavonoid content was observed with higher concentration of ethanol. The flavonoid content reached a maximum of 14.8 mg Qu eq./g sample at pure ethanol. Adding a certain amount of water in ethanol might improve the extracting efficiency (Pan et al., 2001). Therefore, 50% aqueous ethanol concentration in water was selected as the concentration of choice for the rest of extraction experiments.

Fig. 3. Effect of ethanol concentration in water on the extraction of total phenolic contents and total flavonoid contents. Solvent: 100 ml; dried eucalyptus leaves: 5g; MAE for 9 min; liquid/solid ratio: 20:1 ml/g
C. Effect of extraction time on extraction of total phenolic and total flavonoid compounds
A proper study on optimization of extraction time is vital because the extraction time may vary when different parts of the plant are used (Mandal et al., 2007). The contents of total phenolic and flavonoid compounds extracted from eucalyptus leaves at different MAE times are presented in fig 4. The results indicate that the extraction of phenolics and flavonoids was increased with an increase in MAE time. MAE reached the highest point in 15 min. There was no significant difference between 11, 13 and 15 min in MAE of phenolic and flavonoid compounds. The lowest content of phenolic and flavonoid compounds was extracted in 30 s of MAE time. So, MAE time for 11 min was the best condition for the rest of experiments based on MAE.

Fig. 4. Effect of MAE time on the extraction of total phenolic contents and total flavonoid contents. Solvent: ethanol/water (1:1 v/v) 100 ml; dried eucalyptus leaves: 5g; liquid/solid ratio: 20:1 ml/g.
Pan et al. (2003) reported that "MAE of polyphenols and caffeine was found to increase up to 4 min and later decreased with the increase of time". In the extraction of artemisinin an overall 92% extraction was achieved with 12 min after which extraction yield dropped down (Hao et al., 2002). Over exposure may lead to thermal degradation of effective constituents.
D. Effect of liquid/solid ratio on extraction of total phenolic and total flavonoid compounds
Figure 5 indicates that the extraction of phenolic and flavonoid compounds has been increased with an increment in liquid/solid ratio. The liquid/solid ratio (ml/g) 25:1 had no significant difference with 22.5:1 and 20:1 of liquid/solid ratio. Therefore, liquid/solid ratio (ml/g) 20:1 was suitable, and it was used afterwards. Although extraction with higher liquid/solid ratios could extract more phenolic or flavonoid compounds, it would require higher amounts of chemical compounds and energy use. Pan et al. (2000) reported that "high liquid/solid ratio needs more energy and time to treat the leaching solution in their experiments".

Fig. 5. Effect of liquid/solid ratio on the extraction of total phenolic contents and total flavonoid contents. Solvent: ethanol/water (1:1 v/v) 100 ml; MAE for 11 min.
E. Effect of pre-leaching time before MAE on extraction of total phenolic and total flavonoid compounds
Figure 6 shows that the extraction efficiency of phenolic and flavonoid compounds was influenced by pre-leaching time at room temperature before MAE for 11 min. If the pre-leaching time was 120 min, the total phenolic content was increased from 96.6 to 111.8 mg GA eq./g sample, while the flavonoid content was increased from 7.5 to 9.7 mg Qu eq./g sample. Other researchers have also confirmed that pre-leaching has dramatic effect on extraction condition. In a study by Pan et al. (2001), the authors reported that "pre-leaching time at room temperature before MAE for 2 min influenced the percentage extraction of tanshinones, reaching the highest extraction when pre-leaching time of 45 min was allowed". Pan et al. (2003) also reported that "increase in pre-leaching time from 4 min to 90 min increased extraction of polyphenols from green tea leaves by 1.53%, while increased the extraction of caffeine by 0.49%".

Fig. 6. Effect of pre-leaching time at room temperature on the extraction of total phenolic contents and total flavonoid contents. Solvent: ethanol/water (1:1 v/v) 100 ml; dried eucalyptus leaves: 5g; MAE for 11 min; liquid/solid ratio: 20:1 ml/g; room temperature: about 25 °C.
It is obvious that pre-leaching before the MAE is useful for improving the extraction of both phenolic and flavonoid compounds.
F. Effect of UAE time on extraction of total phenolic and total flavonoid compounds
Effects of UAE time on the extraction of phenolic and flavonoid compounds are summarized in Fig. 7. The results indicate that the extraction of phenolic and flavonoid compounds was increased with the augment of UAE. A marked increase of the total phenolic contents was observed up to 15 min.

Fig. 7. Effect of UAE time on the extraction of total phenolic contents and total flavonoid contents. Solvent: ethanol/water (1:1 v/v) 100 ml; dried eucalyptus leaves: 5g; liquid/solid ratio: 20:1 ml/g.
G. Comparison of the efficiency of MAE, UAE and traditional method in extracting total phenolic and flavonoid contents
Table 1 summarizes the efficiencies of extraction using UAE and MAE. These methods are compared with the results of the classic method. The MAE for 5 min extracted the same level of phenolic and flavonoid compounds as compared with UAE for 60 min and the extraction at room temperature for 24 h (p<0.05). The MAE for 11 min combined with pre-leaching for 120 min extracted phenolic and flavonoid compounds about 1.36, 1.75-folds respectively compared with the other two extraction methods. Also, the results show that the time of extraction at room temperature and UAE was about 288 and 12-folds of time of extraction with MAE, respectively. According to the Table 1, MAE can greatly reduce the extraction time for the same conditions of extraction compared with other conventional extraction methods.
Table 1: Comparison of the results of the extraction of MAE, UAE and traditional extraction method. 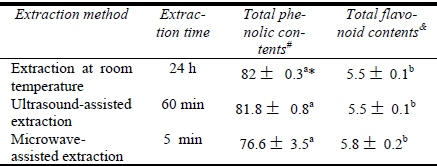
Solvent: ethanol/water (1:1 v/v) 100 ml; dried eucalyptus leaves: 5g; liquid/solid ratio: 20:1 ml/g; room temperature: 25 °C; Ultrasonic extraction temperature: 30-40 °C.
# mg GA eq./ g sample
& mg Qu eq./g sample
* Superscript letters indicate that means with the same letters designation in a column are not significantly different at P < 0.05.
Conventional solvent extraction without the aid of microwave or ultrasonic wave is a time-consuming process based on heat to increase the mass transfer rate in the extraction system. In contrast, microwave-assisted extraction is a fast extraction process where microwave energy is delivered efficiently to materials through molecular interaction with the electromagnetic field and offers a rapid transfer of energy to the extraction solvent and raw plant materials (Zhou and Liu., 2006). Furthermore, based on the findings of other researchers the direct interaction of microwave with solvent also results in the rupture of the plant cells and release of intracellular products into the solvent quickly (Proestos and Komaitis., 2008). For the extraction of tea polyphenols and caffeine from green tea leaves, a 4 min MAE achieved a higher extraction yield than an extraction at room temperature for 20 h, ultrasonic extraction for 90 min and heat reflux extraction for 45 min, respectively (Pan et al., 2003). Shu et al. (2003) also reported that "the extraction yield of ginsenosides from ginseng root obtained by a 15-min MAE (ethanol-water) was higher than that obtained by 10-h conventional solvent extraction (ethanol-water)". Our results are in line with the results of reported studies.
IV. CONCLUSION
Investigating the different methods and conditions for extraction of phenolic and flavonoid compounds from eucalyptus leaves, it was concluded that MAE could be a potential alternative to conventional solid-liquid extraction for the isolation of metabolites, such as phenolic compounds from plants. Compared with traditional extraction and UAE methods, the MAE procedure provided higher extraction yield and selectivity and required relatively shorter times and less intensive treatment and therefore can be proposed for large-scale industrial application in the near future.
ACKNOWLEDGMENTS
The authors would like to acknowledge Gorgan University of Agricultural Sciences and Natural Resources for providing the equipment and supporting this project.
REFERENCES
1. Batish, D.R., H.P. Singh, R.K. Kohli and S. Kaur, "Eucalyptus essential oil as a natural pesticide," Forest Ecology and Management, 256, 2166-2174 (2008). [ Links ]
2. Brooker, M.I.H. and D.A. Kleinig, "Field Guide to Eucalyptus," vol.1. South-eastern Australia, Third edition. Bloomings, Melbourne (2006). [ Links ]
3. Chang, C., M. Yang, H. Wen and J. Chern, "Estimation of total flavonoid content in propolis by two complementary colorimetric methods," J. Food Drug Analaysis, 10, 178-182 (2002). [ Links ]
4. Chen, S.S and M. Spiro, "Study of microwave extraction of essential oil constituents from plant materials," J. Microwave Power Electromagn. Energy, 29, 231-/241 (1994). [ Links ]
5. El-Ghorab, A.H., K.F. El-Massry, F. Marx and H.M. Fadel, "Antioxidant activity of Eucalyptus camaldulensis var. brevirotsris leaf extracts," Nahrung, 47, 41 - 45 (2003). [ Links ]
6. Hao, J., W. Han, S. Huang, D. Xue and X. Deng, "Microwave assisted extraction of artemisnin from Artemisia annua L," Sep. Purif. Technol, 28, 191-196 (2002). [ Links ]
7. Hudson, B. J. F, "Food Antioxidants," Elsevier Applied Science: London (1990). [ Links ]
8. Hwang, J.Y., Y.S. Shue and H.M. Chang, "Antioxidative activity of roasted and defatted peanut kernels," Food Research International, 34, 639-647 (2001). [ Links ]
9. Larson, R.A, "The antioxidants of higher plants," Phytochemistry, 27, 969 -978 (1988). [ Links ]
10. Latha, C, "Microwave assisted extraction of embelin from Embelia ribes," Biotechnol. Lett, 29, 319-322 (2007). [ Links ]
11. Li, H., B. Chen, Z. Zhang and S. Yao, "Focused microwave assisted solvent extraction and HPLC determination of effective constituents in Eucommia ulmodies Oliv," Talanta, 63, 659-665 (2004). [ Links ]
12. Loliger, J, "The use of antioxidants in food," Free Radicals and Food Additives, Eds. O.I. Aruoma, B. Halliwell, Taylor and Francis. London, 129-150 (1991). [ Links ]
13. Mandal, V., Y. Mohan and S. Hemalatha, "Microwave Assisted Extraction - An Innovative and Promising Extraction Tool for Medicinal Plant Research," Pharmacognosy Reviews, 1, 7-18 (2007). [ Links ]
14. Mattina, M.J.I., W.A.I. Berger and C.L. Denson, "Microwave assisted extraction of taxanes from Taxus biomass," J. Agric. Food Chem, 45, 4691-4696 (1997). [ Links ]
15. McDonald, S., P.D. Prenzler, M. Autolovich and K. Robards, "Phenolic content and antioxidant activity of olive extracts," Food Chemistry, 73, 73-84 (2001). [ Links ]
16. Pan, X., H. Liu, G. Jia and Y.Y. Shu, "Microwave assisted extraction of glycyrrhizic acid from licorice root," Biochem. Eng. J, 5, 173-177 (2000). [ Links ]
17. Pan, X., G. Niu and H. Liu, "Microwave assisted extraction of tanshinones from Salvia miltiorrhiza bunge with analysis by high performance liquid chromatography," J. Cromatogr. A, 922, 371-375 (2001). [ Links ]
18. Pan, X., G. Niu and H. Liu, "Microwave assisted extraction of tea polyphenols and tea caffeine from green tea leaves," Chem. Eng. Process, 42, 129-133 (2003). [ Links ]
19. Proestos, C. and M. Komaitis, "Application of microwave-assisted extraction to the fast extraction of plant phenolic compounds," LWT, 41, 652-659. (2008). [ Links ]
20. Rice-Evans, C.A., N.J. Miller, P.G. Bolwell, P.M. Bramley and J.B. Pridham, "The relative antioxidant activities of plant-derived polyphenolic flavonoids," Free Radical Res, 22, 375-383 (1995). [ Links ]
21. Stashenko, E.E., B.E. Jaramillo and J.R. Martinez, "Comparison of different extraction methods for the analysis of volatile secondary metabolites of Lippia alba (Mill.) N.E. Brown, grown in Columbia and evaluation of its in vitro antioxidant activity," J. Chromatogr. A, 1025, 93-103 (2004). [ Links ]
22. Shu, Y.Y., M.Y. Ko and Y.S. Chang, "Microwave assisted extraction of ginsenosides from ginseng root" Microchemical journal, 74, 131-139 (2003). [ Links ]
23. Zhang, F., B. Chen, S. Xiao and S. Yao, "Optimization and comparison of different extraction techniques for sanguinarine and chelerythrine in fruits of Macleaya cordata (Wild) R. Br," Sep. Purif. Technol, 42, 283-290 (2005). [ Links ]
24. Zhou, H and C. Liu, "Microwave assisted extraction of solanesol from tobacco leaves," J. Chromatogr. A, 1129, 135-139 (2006). [ Links ]
Received: October 25, 2011
Accepted: November 16, 2011.
Recommended by subject editor: María Luján Ferreira














