Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Latin American applied research
Print version ISSN 0327-0793
Lat. Am. appl. res. vol.42 no.3 Bahía Blanca July 2012
Calcareous chitin: a novel low-cost sorbent for cadmium (II)
M.S. Rodríguez, M. Zalba, M.T. Goitía, A. Pugliese, A. Debbaudt, E. Agulló, P.C. Schulz and L. Albertengo
Departamento de Química e Instituto de Química del Sur (INQUISUR, CONICET), Universidad Nacional del Sur, Bahía Blanca, Argentina. E-mail: pschulz@criba.edu.ar
Abstract — This research deals on a new low-cost porous sorbent named calcareous chitin (CaCh). CaCh is prepared by alkaline treatment of crustacean exoskeleton to produce a porous matrix of chitin and calcium carbonate free of original proteins. SEM-EDX, X-ray, FT-IR were used to characterize the material. CaCh structure is more porous than that of chitin. Adsorption studies demonstrated its capability to remove Cd(II) from waters. The Langmuir equation fitted the adsorption isotherms well. Due to its easy, rapid and low cost preparation and its adsorption characteristics calcareous chitin can be used as low cost sorbent of heavy metals ions like cadmium for wastewater treatment.
Keywords — Calcareous Chitin; Chitin; Sorption; Cadmium; Low-Cost Sorbent; Wastewater Remediation.
I. INTRODUCTION
Heavy metals in water are a major preoccupation because of their toxicity towards aquatic-life, human beings and the environment. Heavy metals, such as Cd2+, Cu2+, Pb2+, Hg2+ and Zn2+ are toxic to human beings and other living organisms, if their concentration exceeds the tolerance limit. As they do not degrade biologically like organic pollutants, their presence in drinking water or industrial effluents is a public health problem due to their absorption and therefore possible accumulation in organisms. These heavy metals are introduced into natural water resources by wastewater discharged from industries such as smelting, metal plating, Cd-Ni batteries, fertilizers, mining pigments, stabilizers and alloy manufacturing (Rao et al., 2010).
Among toxic heavy metals, cadmium is one of the most dangerous for human health, is a non-essential and non-biodegradable metal which slowly accumulates in the human body, the serious incident of itai-itai disease, which was caused by cadmium poisoning owing to mining in Japan, was related to cadmium ions. The harmful effects of Cd(II) ions are renal damage, hypertension, proteinuria, kidney stone formation and testicular atrophy. Cd(II) ions may replace Zn(II) ions in some enzymes thereby affecting the enzyme activity (Inaba et al., 2005; Teeyakasem et al., 2007). This necessitates the removal of Cd(II) ions from wastewater and water (Kula et al., 2008). However, cadmium has also practical applications: e.g., it is highly corrosion resistant and is used as a protective coating for iron, steel, and copper. The industrial uses of cadmium are increasing in plastics, paint pigments, electroplating, batteries, mining, and alloy industries (Al-Asheh and Duvnjak, 1997, Corami et al., 2008).
Several physical and chemical processes have been studied and developed over the years to remove the heavy metal pollutants from wastewaters at high concentrations. Some of these processes are adsorption, coagulation, flotation, biosorption, chemical precipitation, reverse osmosis, electrolytic recovery, ion exchange, ultra filtration and electrochemical methods. In this process, adsorption can be seen as an efficient and economic method to remove the heavy metal pollutants at low concentrations (Stafiej and Pyrzynska, 2007).
Adsorbent materials from forestry, fishery and agriculture have attracted much attention to several workers. Some of the reported sorbents include peanut hulls (Brown et al.,2000), maize bran (Singh et al., 2006), sawdust (Taty-Costodes et al., 2003), sugar beet pulp (Reddad et al., 2002), crab shell (Vijayaraghavan et al., 2006), cornstarch (Kweon et al., 2001), exhausted coffee (Orhan and Buyukgungor, 1993), rice husk (Kumar and Bandyopadhyay, 2006), orange waste (Dhakal et al. 2005), biological materials including bacteria (Brierley, 1990), fungi (Tsezos and Volesky, 1981), yeast (Volesky et al., 1993), microalgae (Darnall et al., 1986) and chitin (Ghimire Darnall et al., 2001, Benguella and Benaissa, 2002, Copello et al., 2008) just to mention a few in the literature.
Chitin is one of the most abundant organic materials, being only exceeded by cellulose in the amount produced by biosynthesis. It is an important constituent of the exoskeleton in animals, especially in crustacean, mollusks and insects. It is also the principal fibrillar polymer in the cell wall of certain fungi (Eugene and Lee, 2003). The chitin molecule consists of (beta)(1-4)-2-acetamide 2 deoxi-D-glucose units, some of which are deacetylated.
One factor in many of the more traditional applications of chitin is its high chelating capacity. It has been used to remove diverse compounds from waste streams including heavy metals (Muzzarelli, 1973, 1977; Babel and Kurniawan, 2003).
The goal of this paper is to study a new low-cost porous sorbent obtained from crustacean exoskeleton, which is a waste from fishery industry. Preparation, characterization and adsorption studies on its capability in the removal of Cd(II) are reported.
II. METHODS
A. Calcareous Chitin and Chitin preparation
Calcareous Chitin (CaCh) was obtained in the Laboratorio de Investigaciones Básicas y Aplicadas en Quitina (LIBAQ), of INQUISUR (UNS-CONICET) from crustacean exoskeleton waste. The raw material was homogenized and triturated in an industrial crusher (Westinghouse model DASO6). The product was rinsed at room temperature with water in order to remove all organic materials. The clean residue was treated with 9 % (w/w) NaOH (Lab Chem, Inc., Pittsburgh, Pa) at 60 °C for 90 min, to remove proteins.
Chitin (Ch) was obtained by demineralization of CaCh with 10 % (v/v) HCl (Merck, Buenos Aires , Argentina) at 40 °C for 1 h, washed with water at room temperature.
Both sorbents were dried and sieved using a number 70 mesh Fisher Scientific Sieve.
B. Chitin and Calcareous Chitin characterization studies
The protein content was determined by the UV-Visible Spectrophotometric Bradford protein assay (Bradford, 1976). For determining calcium 50 mg of CaCh was treated with 15 mL of HCl 15 % (v/v). After filtering the supernatant was diluted to 100 mL and the calcium content was determined by a UV-Visible Spectrophotometric Cresolphthalein complexometric method (Baginski et al., 1982). The calcium content was then expressed as CaCO3 content since CaCh is formed by an interplay of chitin and calcium carbonate matrixes.
The following studies were done directly with the biopolymers before and after the sorption studies.
Surface area, pore volume and pore size of Ch and CaCh were determined with a BET surface area analyzer nitrogen intrusion porosimeter by means of adsorption of ultra pure nitrogen (-196°C) (Micromeritics' ASAP 2020) Accelerated Surface Area and Porosimetry analyzer uses the gas sorption technique.
Samples were observed and photographed with an optic microscope (Olympus BH-2-UMA with a camera Sony CCD IRIS/RGB).
A JEOL JSM - 35 CF scanning electron microscope was used to characterize the surface of particles. The samples were prepared by gold coating using a sputter coater (Pelco 91000).
Microanalysis (EDX) was made with EDX DX - 4, with a 0.1 % w/w detection range from B (Boron) to U (Uranium).
IR Spectra were recorder on a Nicolet - Nexus 470 FT-IR spectrometer on translucent disk obtained by pressing the ground material with KBr (1% w/w).
X-ray diffraction data were collected using a Rigaku X-ray diffractometer D-Máx. III- C, 35 kV and 15 mA, with Cu Kα radiation and a monochromator of curved single crystal (graphite).
C. Cadmium sorption studies
For cadmium sorption studies, each sorbent (50 mg) was placed in 15 mL of NaClO4 solution with different concentrations (10-1; 10-2 and 10-3 M). The range of pH was 3 to 7, and different concentrations of Cd(II) (0-474 mg/L) from a solution (2.840 mg/L) prepared with Cd(NO3)2• 4H2O (Windor Laboratories Limited) were added. The concentrations were determined with a Perkin-Elmer Analyst 200 Atomic Absorption Spectrophotometer. The mixture was stirred using a magnetic horizontal stirrer for 15, 30, 45, 60 and 90 min. Work temperature was kept at 20 ± 1°C. The sorbent was removed from the solution by filtration through a sintered glass filter. This method was used to study the effects of pH, ionic strength, and the kinetic process. Adsorption experiments were done in triplicate.
The initial and residual solution metal concentrations were measured and the metal uptake q (mg ion metal/g sorbent) was determined as follows (Saha et al., 2005).
q = (C0−Ct)×V/m
where C0 and Ct are the initial and final metal ion concentrations (mg/L), respectively, V is the volume of solution (mL), and m is the sorbent weight (g) in dry form.
Freundlich and Langmuir adjustments were applied.
The calcium content released was determined by a UV-Visible Spectrophotometric Cresolphthalein complexometric method (Baginski et al., 1982).
III. RESULTS AND DISCUSSION
A. Ch and CaCh Characterization
The Ch and CaCh characterization data are: Protein content < 1 g % in both compounds; Ca(II) content Ch : < 0.1 g %, CaCh 21.2 g %; CaCO3: Ch : undetected, CaCh: 53 g %.
In the nitrogen adsorption determinations the following results can be drawn. Both materials showed adsorption isotherms Type IV with its hysteresis loop (Fig.1), which is associated with capillary condensation taking place in mesopores, and with the limiting uptake over a range of high p/p°.

Fig. 1. Adsorption isotherm for CaCh, o: sorption,  : desorption
: desorption
The initial part of the Type IV isotherm is attributed to monolayer-multilayer adsorption since it follows the same path as the corresponding part of a Type II isotherm obtained with the given adsorptive on the same surface area of the adsorbent in a non-porous form. The hysteresis loop is H3 type, which is characteristic of slit-shaped pores. Type IV isotherms are given by many mesoporous industrial adsorbents (Sing et al., 1985).
Figure 2 shows the pore radius distribution for Ch and CaCh. It can be seen that CaCh has mainly pores centered in R ≈ 1.91 nm (but ranging from 1.53 to 2.13 nm), and a few with R ≈ 2.38 nm. Ch has few pores with R ≈ 0.68 - 0.86 (inset fig.2) and 2.38 nm, and a large proportion that starting at about 2,6 nm and has maximum at R ≈ 3.06 nm.

Figure 2. Distribution of pore radius for Ch (---) and CaCh (-). Inset: amplification of the low R region for chitin, showing the minimum pore radius (~ 0.68 nm).
Other results of this study are summarized in Table 1. From both the pore distribution curve and the Barret-Joyner-Halenda (BJH) (Barrett et al., 1951) calculation, we can observe that CaCh has mesopores larger than those of Ch, which shows some micropores. Moreover, Ch pores seem to be less defined and probably are slits between chitin lamellae, with no defined width, whilst those of CaCh seem to be well-defined pores having a Gaussian size distribution. Both BET and BJH analysis gave specific surface area and pore volume consistently higher for CaCh than for Ch.
Table 1 Chitin and Calcareous Chitin porosity data 
B. Microscopic characterization
The Ch structure is crystalline with compact and smooth surface (Fig. 3) when it is observed by optic microscopy. This is compatible with the interpretation of slit pores as inter-lamellae separation gaps. CaCh exhibits rough and irregular surface with separately calcium carbonate crystals, which would indicate that two different structures constitute the sorbent that consists in a mix of chitin and calcium carbonate (Fig. 4).

Figure 3. Optical photomicrograph of chitin. The bar represents 250 mm

Figure 4. Optical photomicrograph of calcareous chitin. The bar represents 150 mm
C. SEM
Scanning electron microscopy was used to study the particle size and surface morphology. Figure 5 shows a micrograph of Ch that is crystalline, compact and laminar. In Fig. 6, CaCh shows a rough and irregular surface with pores of chitin and calcium carbonate. The higher porosity and surface area of CaCh when compared with those of Ch may be caused by differences in the preparation method. The alkaline method to prepare CaCh from crustacean exoskeletons produces a porous material formed by chitin and calcium carbonate, while the acidic preparation of Ch produced a smooth, nonporous surface.

Figure 5. SEM microphotograph of chitin fraction and its EDX spectrum. (200 X, the bar represents 100 mm).

Figure 6. SEM microphotograph of calcareous-chitin fraction and its EDX spectrum. (200 X, the bar represents 100 mm).
D. EDX
Ch and CaCh EDX spectra (Figs. 5-6) show that both materials have calcium carbonate signals but in different concentration (Ch, 1.59% w/w; CaCh, 8.78% w/w). The peak at 2.12 without denomination corresponds to the Au conductive coating layer.
E. IR spectroscopy
Comparison between CaCh and Ch FT-IR spectra (Fig. 7) confirms that CaCh is a mix of Ch and Ca(II) carbonate, because the only difference with Ch spectrum is the presence of carboxylate bands (COO-).

Figure 7. FT-IR Spectra of a) calcareous chitin and b) chitin.
It can be concluded that the polymer consists in a mix of two different structures without linkages (1740 -1690 cm-1, stretching carbon-oxygen double bond; 1300-1100 cm-1 and 1100-1000 cm-1 corresponding stretching carbon-oxygen simple bond) (Brugnerotto et al., 2001)
F. X-ray diffraction spectrometry
X-ray diffraction analysis (Fig. 8 a and b) of Ch show two peaks at 2θ = 9.3° and 19.4° (4.6  ). In the ChCa diffractogram, the same peak is seen, with lower intensity due to the predominance of calcite at 2θ = 29.4° (3.03
). In the ChCa diffractogram, the same peak is seen, with lower intensity due to the predominance of calcite at 2θ = 29.4° (3.03  ) (Rinaudo, 2006).
) (Rinaudo, 2006).

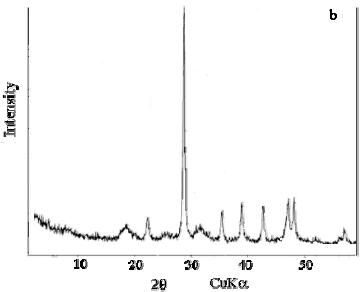
Figure 8. Representative X-ray diffraction patterns of (a) chitin and (b) calcareous chitin.
G. Cd(II) adsorption study
Cadmium (II) was selected for adsorption studies to evaluate the potential of CaCh to remove heavy metal pollutants. Figure 9 (SEM) shows that the surface of CaCh after Cd (II) adsorption appears unchanged, remaining compact; rough, irregular and porous. EDX analysis (Fig. 9) reveals the Cd(II) signal on CaCh surface. Ca(II) concentration decreases from 8.78 % w/w (Fig. 7) to 4.55% w/w and that of Cd(II) reaches 2.36 % w/w, but there is no evidence about the type of retention. X-ray diffraction spectrum of CaCh-Cd (Fig.10) shows reflections at 3.79  2.95
2.95  and 2.07
and 2.07  corresponding to CdCO3 and a calcite peak at 2q = 29.4° (3.03
corresponding to CdCO3 and a calcite peak at 2q = 29.4° (3.03  ).
).

Figure 9. SEM microphotograph of calcareous chitin (CaCh) after Cd(II) adsorption and its EDX spectrum. (200 X, the bar represents 100 mm).

Figure 10. XRD spectrum of calcareous chitin after Cd(II) sorption.
H. Kinetic study
The kinetics of Cd(II) uptake on CaCh was investigated. As shown in Table 2, Cd(II) uptake was rapid. The adsorption occurred primarily within 15-30 min and equilibrium was achieved. For subsequent experiments, adsorption tests were fixed at 60 min.
Table 2: Kinetics of cadmium adsorption by CaCh at 25°C and pH 5.5. Initial concentration of metal 150 mg.L-1 
H. Effect of ionic strength on adsorption
The sorption data shown in Table 3 show that at the concentrations of NaClO4 and Cd (II) studied, there is no significant influence of the ionic strength in the cation adsorption, so the NaClO4 10-2 M concentration was used in the later experiences.
Table 3: Effect of the ionic strength on Cd (II) sorption by CaCh 
I. Influence of pH on Cd adsorption
According to species distribution, Cd(II) exhibits only the +2 valence in aqueous solution below pH = 8.0. Above this value, mononuclear hydrolysis products appear, but the low solubility of the hydroxide limits the Cd(II) concentration (present as CdOH+ and Cd(OH)2) to values minor to 10-5 M until reaching pH = 13 (Baes and Mesmer, 1976). Below pH = 4, calcium carbonate of the CaCh counterfoil is dissolved. So, the range of pH used in this study was between 4 and 7. Table 4 shows the relation between Cd(II) adsorbed quantity (q), and calcium(II) released (Ca(II)r), when C0 of Cd(II) was 150.77mg/L. In the same table it can be seen that at pH = 3 the quantity of Ca(II) released to the solution was the highest. This quantity decreased and remained constant in the pH = 4 to 7 range. Also the percentage of adsorption in this pH range was maximum and constant. Even at different initial pH values, the final pH at equilibrium was 7.6-7.7. This is attributed to the balance between the calcium carbonate released of CaCh and the carbon dioxide dissolved in water:
Ca CO3 + H2O(l) ↔ Ca++(aq)+ CO2(aq) + 2OH-(aq)
Table 4: Relation between Cd(II) adsorbed quantity and Ca(II) released at different pH values. 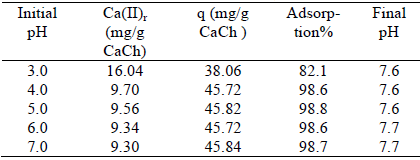
This pH is achieved simply with CaCh in water, without the addition of Cd(II).
J. Cd(II) Retention
Table 5 shows the correlation between Cd(II) adsorbed by Calcareous Chitin, quantity (q) , calcium(II) released (Ca(II)r) and Cd(II) retention by Chitin. The maximum quantity of adsorbed metal, qmax, was 71.4 mg Cd/g CaCh and the maximum Ca released was 17.8 mg Ca/g CaCh. The errors were computed with the Student t distribution and a confidence level of 0.1.
Table 5 Cd(II) retention (q) and Calcium released by Calcareous Chitin and Cd(II) retention by Chitin. 
The molar retention was 0.618 mol Cd/g CaCh. For larger quantities of Cd(II) in solution, Cd adsorption and Ca(II) release remained practically constant, diminishing therefore the percentage of Cd(II) retention.
Figure 11 shows the adsorption capacity (q) of CaCh and Ch, respectively. It can be seen that the adsorption capacity of CaCh (69.2 mg/g) is 2.3 times higher than that of chitin (29.5 mg/g), which exhibits a molar retention of 0.263 mol Cd/g, lower than CaCh retention (0.618 mol Cd/g).

Figure 11. Comparative Cd(II) adsorption capacity of chitin and calcareous chitin.
The increased retention capacity of CaCh when compared with that of Ch may be caused by a combination of higher specific surface area of the former (which in turn is related to tits higher porosity), and ion exchange between Ca(II) and Cd(II).
K. Adsorption isotherms
Several models to describe experimental data of adsorption isotherms have been published in the literature. The Langmuir and Freundlich models are most frequently applied to analyze the sorption of cations from solutions on solid surfaces. In this work, both models were used to describe the relationship between the amount of Cd(II) ion adsorbed and its equilibrium concentration in solution. Table 6 shows the Langmuir and Freundlich parameters found.
Table 6: Parameters of Langmuir and Freundlich isotherms for Chitin and Calcareous Chitin 
The R2 values (> 0.90) show that both models can adequately describe the adsorption data. However, the Langmuir equation shows a better fit, and the experimental qmax (69.2 mg/g) has no significant difference with that obtained by extrapolation and agrees with previously published works for this type of adsorbent (Debbaudt et al., 2004; Ly et al., 2009).
Results of other sorbents used to the elimination of Cd(II) are qmax = 76.4 mg/g for the fibrous chelating ion exchanger FIBAN-X1 (Soldatov et al., 2011); while Vinod et al. (2009) found qmax = 59.7 mg/g for a natural carbohydrate of India. For the adsorption of Cd(II) on activated carbon, the following results at 303 K: KL = 0.358 L/mg, qmax=1.670 mg/g; KF= 0.444 (mg/g)(1/g)1/n, 1/n = 0.462 were found (Kula et al., 2008). Comparison with results in Table 6 shows that CaCh sorption characteristics are good.
Consequently, it can be deduced that cadmium-CaCh adsorption probably occurs in the unilayer, as Benguella and Benaissa (2002) demonstrated for chitin.
III. CONCLUSIONS
Calcareous chitin possess an effective sorption capacity for Cd(II) ions about 2.3 times greater than chitin. This is due to the synergistic adsorption capacity of the chitin and calcium carbonate in the mixed matrix and, moreover, due to the higher specific area related to its porosity. The optimal working pH range is between 4 to 7, which is limited by calcium release below pH = 4 and Cd(II) precipitation above pH = 7.
The calcareous chitin has then potential application as a sorbent of heavy metal ions like cadmium for wastewater treatment. Its preparation is easy, rapid and low-cost, giving a material with excellent adsorption properties obtained from waste material from fishery.
The cost of the CaCh is estimated between 5 to 7 dollars per kg, due to the abundance of raw materials and the minimum handling processing without risks (NaOH 4% which may be recycled, 60°C, 45 min).
REFERENCES
1. Al-Asheh, S. and Z. Duvnjak, "Sorption of cadmium and other heavy metals by pine bark," J. Hazard. Mater. 56,35-51 (1997). [ Links ]
2. Babel, S. and T.A. Kumiawan, "Low-cost adsorbents for heavy metals uptake from contaminated water: a review," J. Hazard. Mater., 97, 219-243 (2003). [ Links ]
3. Baes, C.H.F. and R.E. Mesmer, The hydrolysis of cations, Wiley, New York (1976). [ Links ]
4. Baginski, E.S., S.M. Slawa and B. Zak, "Phosphate, Inorganic Selected Methods of Clinical Chemistry. 9: Selected Methods for the Small Chemistry Laboratory," Amer. Assoc. Clin. Chem.,Washington DC. 313-316 (1982) [ Links ]
5. Barrett E. P, Joyner L.G. and Halenda P.P., "The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms", J. Am. Chem. Soc. 73, 373-380 (1951). [ Links ]
6. Benguella, B. and H. Benaissa, "Effects of competing cations on cadmium biosorption by chitin," Colloid Surf. A: Physicochemical and Engineering Aspects, 201, 143-150 (2002). [ Links ]
7. Bradford, M.M., "A rapid and sensitive for the quantitation of microgram quantities of proteim utiling the principle of protein dye Bending," Anal. Biochem. 72, 248-254 (1976). [ Links ]
8. Brierley, C.L., "Metal immobilization using bacteria," In: H.L. Ehrlich, C.L. Brierley (Eds.), Microbial Mineral Recovery, McGraw-Hill. , New York 303-324 (1990). [ Links ]
9. Brown, P., I.A. Jefcoat, D. Parrish, S. Gill and E. Graham, "Evaluation of the adsorptive capacity of peanut hull pellets for heavy metals in solution," Adv. Environ. Res., 4, 19-29 (2000). [ Links ]
10. Brugnerotto, J., J. Lizardi, F.M. Goycoolea, W. Arguelles Monal, J. Desbrieres and M. Rinaudo, "An Infrared investigation in relation with chitin and chitosan characterization," Polymer. 42, 3569-3580 (2001). [ Links ]
11. Copello, G.J., F. Varela, R. Martínez Vivot and L.E. Díaz, "Immobilized chitosan as biosorbent for the removal of Cd(II), Cr(III) and Cr(VI) from aqueous solutions," Biores. Technol. 99, 6538-6544 (2008). [ Links ]
12. Corami, A., S. Mignardi and V. Ferrini, "Cadmium removal from single- and multi-metal (Cd+Pb+Zn+ Cu) solutions by sorption on hydroxyapatite," J. Colloid Interface Sci., 317, 402-408 (2008). [ Links ]
13. Darnall, D.W., B. Greene, M.T. Henzi, J.M. Hosea, R.A. McPherson, J. Sneddon and M.D. Alexander, "Selective recovery of gold and other metal ions from an algal biomass," Environ. Sci. Technol.. 20, 206-208 (1986). [ Links ]
14. Debbaudt, A.L., M.L. Ferreira and M.E. Schaider, "Theoretical and experimental study of M2+ adsorption on biopolymers III comparative kinetic pattern of Pb, Hg and Cd," Carbohydr. Polym. 56, 321- 332 (2004). [ Links ]
15. Dhakal, R.P., K.N. Ghimire and K. Inoue, "Adsorptive separation of heavy metals from an aquatic environment using orange waste," Hydromet. 79,182-190 (2005). [ Links ]
16. Eugene, K. and Y.L. Lee, "Implantable applications of chitin and chitosan," Biomaterials, 24, 2339-2349(2003). [ Links ]
17. Ghimire, K.N., K. Inoue, T. Miyajima, K. Yoshizuka and T. Shoji, "Adsorption of some metal ions and mineral acids on chitin," Chitin Chitin Res., 7,61-68 (2001). [ Links ]
18. Inaba, T., E. Kobayashi, Y. Suwazono, M. Uetani, M. Oishi, H. Nakagawa and K. Nogawa,"Estimation of cumulative cadmium intake causing Itai-itai disease," Toxicol. Lett.159, 192-201 (2005)- [ Links ]
19. Kula, I., M. Uğurlu, H. Karaoğlu and A. Çelik, "Adsorption of Cd(II) ions from aqueous solutions using activated carbon prepared from olive stone by ZnCl2 activation," Biores. Technol., 99, 492-501 (2008). [ Links ]
20. Kumar, U. and M. Bandyopadhyay, "Sorption of cadmium from aqueous solution using pretreated rice husk," Biores. Technol., 97, 104-109 (2006). [ Links ]
21. Kweon, D.K., J.K. Choi, E.K. Kim and S.T. Lim, "Adsorption of divalent ions by suecinylayted and oxidized cornstarch," Carbohydr. Polym. 46, 171-177 (2001). [ Links ]
22. Ly, K.L., Y.L. Du and C.M. Wang, "Synthesis of carboxilated chitosan and adsorption properties for cadmium (II), lead (II) and copper (II) from aquous solutions," Water Science & Techn., 60, 467-474 (2009). [ Links ]
23. Muzzarelli, R.A.A., Natural Chelating Polymers, Pergamon, Oxford (1973). [ Links ]
24. Muzzarelli, R.A.A., Chitin, Pergamon, Oxford (1977). [ Links ]
25. Orhan, Y. and H. Buyukgungor, "The removal of heavy metals by using agricultural wastes," Water Sci. Technol. 28, 247-255 (1993). [ Links ]
26. Rao, K.S., M. Mohapatra, S. Anand and P. Venkateswarlu, "Review on cadmium removal from aqueous solutions,"International Journal of Engineering, Science and Technology, 2, 81-103 (2010). [ Links ]
27. Reddad, Z., C. Gerente, Y. Andres and P.L. Cloirec, "Adsorption of several metal ions onto a low-cost biosorbent: kinetic and equilibrium studies," Environ. Sci. Technol., 36, 2067-2073 (2002). [ Links ]
28. Rinaudo, M., "Chitin and chitosan: Properties and applications," Progress in Polymer Science, 31, 603-632 (2006). [ Links ]
29. Saha, T.P., S. Karmaker, H. Ichikawa and Y. Fukumori, "Mechanisms and kinetics of trisodium 2-hydroxy-1,1 azonaphtalene-3,4,6-trisulfonate adsorption onto chitosan," J. Colloid Interface Sci., 286, 433-439 (2005). [ Links ]
30. Sing, K.S.W., D.H. Everett, R.A.W. Haul, L. Moscou, R.A. Pierotti, J. Rouquerol and T. Siemieniewska, "Reporting Physisorption data for gas/solid systems with Special Reference to the Determination of Surface Area and Porosity," Pure Appl. Chem. 57, 603-619 (1985). [ Links ]
31. Singh, K.K., M. Talat and S.H. Hasan, "Removal of lead from aqueous solutions by agricultural waste maize bran," Biores. Technol., 7, 2124-2130 (2006). [ Links ]
32. Stafiej, A. and K. Pyrzynska, "Adsorption of heavy metal ions with carbon nanotubes," Sep. Purif. Technol., 58, 49-52 (2007). [ Links ]
33. Soldatov, V.S., V.M. Zelenkovskii and L.A. Orlovskaya, "Sorption of bivalent ions by a fibrous chelating ion exchanger and the structure of sorption complexes," React. & Funct. Polym., 71, 49-61 (2011). [ Links ]
34. Taty-Costodes, V.C., H. Fauduet, C. Porte and A. Delacroix, "Removal of Cd(II) and Pb(II) ions from aqueous solutions by adsorption onto sawdust of Pinus Sylvestris," J. Hazard. Mater., B105, 185-195 (2007). [ Links ]
35. Teeyakasem, W., M. Nishijo, R. Honda, S. Satarug, W. Swaddiwudhipong and W. Ruangyuttikarn,"Monitoring of cadmium toxicity in a Thai population with high-level environmental exposure," Toxicol. Lett, 169, 189-190 (2007). [ Links ]
36. Tsezos, M. and B. Volesky, "Biosorption of uranium and thorium," Biotechnol. Bioeng., 23, 583-604 (1981). [ Links ]
37. Vijayaraghavan, K., K. Palanivelu and M. Velan, "Biosorption of copper(II) and cobalt(II) from aqueous solutions by crab shell particles," Biores. Technol. 97, 1411-1419 (2006). [ Links ]
38. Vinod, V.T.P., R.B. Sashidhar, B. Sreedhar, B. Rama Rao, T. Nageswara Rao and J.T. Abraham, "Interaction of Pb+2 and Cd+2 with gum kondagogu (Cochlospermum gossypium). A natural carbohydrate polymer with biosorbent properties," Carbohydr. Polym., 78, 894-901 (2009). [ Links ]
39. Volesky, B., H. May and Z.R. Holan, "Cadmium biosorption by Saccharomyces cerevisiae," Biotechnol. Bioeng., 41, 826-829(1993). [ Links ]
Received: July 20, 2011
Accepted: October 27, 2011
Recommended by subject editor: Mariano Martín Martín














