Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Latin American applied research
versión impresa ISSN 0327-0793
Lat. Am. appl. res. vol.42 no.4 Bahía Blanca oct. 2012
ARTÍCULO
Synthesis of docosanoic and tetracosanoic acid from alcohols using a task-specific ionic liquid catalyst
N. Guajardo and C. Carlesi
Escuela de Ingeniería Química, Pontificia Universidad Católica de Valparaíso, Avenida Brasil 2147, 2362804 Valparaíso, Chile
carlos.carlesi@ucv.cl
Abstract Catalytic batch oxidation of docosanol to docosanoic acid and tetracosanol to tetracosanoic acid was performed using hydrogen peroxide as oxidant and a functionalized ionic liquid composed of an Aliquat® cation and a peroxotungstophosphate anion acting as catalyst.
Conversion of 76% and selectivity of 60.2% were obtained after 6 hours for docosanol oxidation at optimal operational conditions (temperature of 90°C; total-H2O2/alcohol molar ratio = 3; alcohol/catalyst mass ratio = 300). In the case of tetracosanol oxidation 85% conversion and 68% selectivity were reached in the same time interval at optimal operational conditions (temperature of 90°C; total- H2O2/ alcohol molar ratio = 5; alcohol/final-catalyst mass ratio = 50). In both cases the oxidant and the catalyst were continuously added throughout the reaction time, thus enabling continuous reaction performance.
The batch results obtained in this study confirm the technical feasibility of operating a process to produce both these high molecular acids from alcohols as renewable raw materials.
Keywords Docosanol; Docosanoic Acid; Tetracosanol; Tetracosanoic Acid; Quaternary Ammonium Peroxotungstophosphate Catalyst; Ionic Liquid.
I. INTRODUCTION
Traces of docosanoic and tetracosanoic acid are found in vegetable oils from different sources, reaching a maximum content of 1% in peanut oil (Beare-Rogers et al., 2001) and in peanut hulls (Lee, 2008). Docosanoic acid has natural humectants and smoothing properties that are therefore of particular interest to the cosmetics and pharmaceutical industry, as indicated by US agricultural department research (Lee, 2008; Coupland and Smith, 1989). The use of docosanoic acid (a 22 carbon chain acid) in cosmetic applications has been seen extensively due to its commercial availability (Lee, 2008). On the other hand, tetracosanol is not produced on an industrial scale at the present, and the natural sources from which it could be extracted have a high added value themselves, therefore extraction is not cost effective. Among the renewable sources of tetracosanol we can find: the red sandalwood tree (Adenanthera pavonina), for which concentration can reach a maximum of 25% (Markley, 1960), carnauba wax (average content of 30%) and rice bran wax (average content of 40%) (Markley, 1960; Beare-Rogers et al., 2001).
Catalytic oxidation of alcohol has been studied to obtain these valuable types of acid (Venturello and Gambaro, 1991; Bortolini et al., 1986; Bi et al., 2001; Kan et al., 2001; Hong et al., 2004). This synthetic route could be of industrial interest as it involves the use of potentially renewable and widely available natural resources in the form of long chain alcohols such as wax from sugar cane, aliphatic alcohols from tail oils, and mixed synthetic alcohols generated in the oxidation of paraffin (Guajardo, 2007; Markovits et al., 2009).
The present paper deals with the study of the oxidation of both docosanol and tetracosanol, using a peroxotungstate functional group of an Ionic Liquid as catalyst.
The reaction medium includes the alcoholic substrate (alcoholic phase) and an aqueous phase containing the oxidant (hydrogen peroxide). The catalyst acts as active oxygen transfer between the two phases of the reaction system. In this case the catalyst belongs to the family of ionic liquids, a family of low temperature molten salts which offer properties (particularly low vapor pressure and high oxidative resistance) that allow efficient separation and recycling of the catalyst when working in highly oxidative aqueous media.
These and other tunable properties of ionic liquids solvents render them a key operative factor for many industrial applications (Plechkova and Seddon, 2008).
II. MATERIALS AND METHODS
A. Chemical reagents
The following reagents were used as purchased without further purification: Sodium sulphate from Scharlau (code SO0665), Phosphoric acid, dichloromethane and hydrogen peroxide from Merck (codes 815058, 106051 and 386790 respectively), Docosanol (95%) from Sasol and docosanoic acid (98%) from Aldrich, Sodium tungstate dihydrate (Na2WO4*2H2O), methyltricaprylammonium chloride {(C8H17)3CH3N+ Cl-} (Aliquat 336), phosphotungstenic acid (PTA) (H3PW12O40), tungstosilisic acid (H4SiW12O40), and tetrabutylammonium hexafluorophosphate (TBA) from Sigma.
B. Reactor set-up
The oxidation tests were carried out in a batch mode, using a thermostated reactor (Ika Eurosta, maximum volume of 2 liters), with mechanical stirring and in vapor reflux mode. The reactor was first loaded with the alcohol (docosanol or tetracosanol) followed by the addition of the aqueous phase containing the catalyst, thus forming a two phase system. The reaction starts with the addition of the oxidant drop by drop. The reaction volume was 500 ml. Samples were collected regularly and then analyzed by gas chromatography (GC HP 6890) in order to follow the reaction paths. Molar conversion (C) and selectivity (S) were calculated using expressions 1 and 2, respectively.
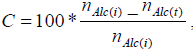 | (1) |
 | (2) |
where nAlc(t), n Alc(i) and nAc(t) represent the molar concentration of the alcohol at reaction time (t), the initial concentration of the alcohol and the concentration of the acid over time.
The catalyst was recovered from the organic phase through distillation (at the bottom) at the end of each run. This is due to the large difference in the vapor pressure of the ionic liquid catalyst in relation to the reaction products and un-reacted reactants.
C. Catalyst synthesis
The ionic liquid catalyst (Acronym: Q3+PW-3) was prepared by replacing the chloride anion of the Aliquat 336 reagent with peroxotungstophosphate anion (PW-3), following the procedure reported by Venturello and D'Aloisio (1988): 90 g sodium tungstate dihydrate (Na2WO4*2H2O) as tungsten precursor was diluted into 252 ml of hydrogen peroxide solution (8% V/V) forming a suspension. This was then heated at 60°C and stirred until a color transition took place (from pale yellow to white); this is an indication of the oxidation reaction. The solution was then cooled and filtered and 22.33 ml of H3PO4 solution (40% V/V) was added to the solid retained in the filter and completed to a volume of 1080 mL with distilled water. This prepared solution was then mixed with a second solution composed of 75.3 g of Aliquat 336® dissolved in 440 ml of dichloromethane. The mixture of solutions was vigorously stirred for 15 minutes and left stand until two liquid phases were formed. After separation the organic phase containing the catalyst was dried over sodium sulphate, and the dichloromethane solvent was then extracted by means of a molecular distiller (KDL5 UIC) operated at 55°C. The obtained solid was analyzed by FT-IR spectroscopy (Perkin Elmer - Spectrum One).
III. RESULTS AND DISCUSSION
A. Catalysis characterization
The principal signals from the FT-IR analysis were (wavelength in cm-1): 3677.14; 3391.60; 2955.84; 2925.01; 2855.16; 1637.53; 1467.03; 1378.04; 1120.15; 1079.84; 1040.77; 940.30; 889.67; 852.46; 811.86; 756.79; 726.06; where the absorbance related directly to the Q3+PW-3 catalyst corresponds to: 889.67 cm-1 (W-O-W); and the zone between 1079.84 and 1040.77 cm-1 is related to (P-O) vibration; the 852.46 cm-1 signal is related to (O-O) and the signal at 940 cm-1 corresponds to (W-O) bond (Alizadeh and Tayebee, 2005; Aubry et al., 1991). This confirmation of the expected absorption pecks indicates the presence of the desired chemical structure of the catalyst.
B. Batch oxidation runs
A series of oxidation runs were performed while monitoring the operative variables: reaction temperature, mass ratio (alcohol/ Q3+PW-3 catalyst) and the molar ratio (H2O2/alcohol).
The most representative results are summarized in Table 1 for the case of docosanol oxidation as regards variation of operative conditions. It can be seen that the best performance is found at a temperature of 90°C; molar ratio H2O2/docosanol equal to 3, and mass ratio docosanol/catalyst equal to 300. The experimental evidence indicates that increases in the peroxide concentration do not produce a significant improvement either in the conversion or in selectivity; this may be related to hydrogen peroxide decomposition phenomena. On the other hand, a further increment in the relative amount of catalyst (beyond the aforementioned optimum ratio) does not improve the oxidation performance, which may be related to the scope of the maximum concentration of hydrogen peroxide beyond which the control of the kinetics becomes independent of the catalyst's relative amount.
Table 1: Summary of results for docosanol oxidation, under different operational conditions, after 6 h of reaction.
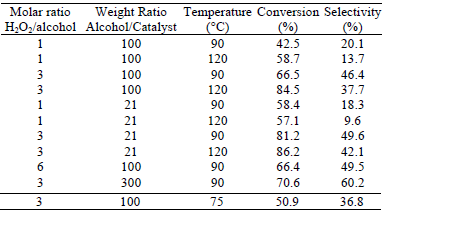
Figure 1 shows a selected result of the oxidative behavior of docosanol. The conversion after six hours of reaction is in the range of 80% in the best operational conditions. A remarkable effect of the means of the addition of the oxidant was shown, where continuous addition of the catalyst to the reaction volume allows an enhancement of both conversion and selectivity parameters by at least 6%, this may be due to catalyst inactivation phenomena.
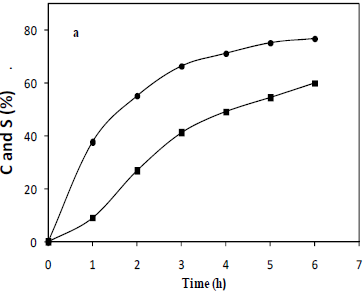
Fig. 1: Representative behavior of conversion (circles) and selectivity (squares) parameters (percentage) in the oxidation of docosanol to docosanoic acid. Temperature 90°C, weight ratio, alcohol/catalyst: 300, molar ratio, H2O2/alcohol: 3.
With regard to the addition of the oxidant, hydrogen peroxide, continuous addition during the reaction also benefits the reaction performance, probably because the thermal and autocatalytic degradation of the peroxide is partially avoided.
The molar and weight ratios shown correspond to the final ones; this is, considering the total oxidant or catalyst added by the end of the batch reaction.
The experiments with tetracosanol were performed considering the optimal range of operational parameters found during docosanol oxidation; the temperature of all runs was controlled at 90°C. Conversion and selectivity to tetracosanoic acid is shown in Fig. 2.

Fig. 2: Representative behavior of conversion (circles) and selectivity (squares) parameters (percent) in the oxidation of tetracosanol to tetracosanoic acid. Temperature 90°C, weight ratio, alcohol/catalyst: 100, molar ratio, H2O2/alcohol: 5.
In Fig. 3, an improvement in reaction performance can be observed when the relative amount of catalyst is raised to more than 90% of conversion after seven hours of operation. As in the case of docosanol oxidation the performance is better when the catalyst is added continuously over the reaction time.

Fig. 3: Representative behavior of conversion (circles) and selectivity (squares) parameters (percent) in the oxidation of tetracosanol to tetracosanoic acid. Temperature 90°C, weight ratio, alcohol/catalyst: 50, molar ratio, H2O2/alcohol: 5.
The results show that the variation in the ratios observed between the catalyst and the alcoholic substrate does not have a proportional effect on the performance of the reaction. This may be due to rapid regeneration of the catalyst. On the other hand, the relative amount of oxidant (H2O2) does have a marked effect on the performance of the reaction.
After verifying the technical feasibility of the proposed reactions and the quantitative separation and recycling of the catalyst, a basic process scheme was proposed, as presented in Fig. 4, for the production of the high molecular aliphatic acids from the corresponding alcohols. Each principal component of the process is specified in the caption of the Fig. 4 (adapted from Markovits et al., 2009).

Fig. 4: Process scheme for oxidative synthesis of docosanoic and tetracosanoic acids from their alcohols. (1) pure aliphatic alcohol (2) aqueous phase, containing the hydrogen peroxide and the catalyst (3) continuous stirred reactor (4) reaction products (5) phase separator (6) aqueous phase (7) organic phase (8) NaOH solution (9) soaping reactor (saponification) (10) saponification reaction products (11) evaporator (12) aqueous purge (13) concentrate (14) distiller (15) bottom purge of low volatile compounds (residue) (16) distillate stream (17) acidifier tank (18) acid aqueous solution (19) acidified stream (20) phase separator (21) solid product (docosanoic or tetracosanol acid) (22) acid aqueous waste.
IV - CONCLUSIONS
The oxidation reaction of docosanol and tetracosanol to docosanoic and tetracosanoic acid was studied with the aim of identifying an appropriate oxidant and catalyst to perform a bi-phasic batch process. Peroxotungstophosphate can actively transfer the active oxidant oxygen from the aqueous to the organic phase. This catalyst was rendered available for the reaction in its anionic form as an ionic liquid. This functionalized ionic liquid is composed of a tri-caprylmethylammonium cation (commercial Aliquat® cation). The efficient performance of this kind of reaction is assured by the good chemical stability of the IL when exposed to high oxidizing media.
76% conversion and 60.2% selectivity were obtained after 6 hours for docosanol oxidation under optimal operational conditions (temperature 90°C; molar ratio of total-H2O2/alcohol = 3; mass ratio of alcohol/catalyst = 300). In the case of tetracosanol oxidation 85% conversion, 68% selectivity were attained in the same time interval under best operational condition (temperature 90°C; molar ratio of total-H2O2/alcohol = 5; mass ratio of alcohol/final-catalyst = 50). Both the oxidant and the catalyst should be added continuously throughout the reaction, thus enabling continuous reaction performance.
ACKNOWLEDGMENTS
The financial support from DII-PUCV "proyecto grupal EIQ" is gratefully acknowledged; N. Guajardo gratefully recognizes the M.Sc. in Chem. Eng. grant financed by PUCV.
REFERENCES
1. Alizadeh, M.H. and R. Tayebee, "Catalytic Oxidation of Aniline by Aqueous Hydrogen Peroxide in the Presence of some Heteropolyoxometalates," J. Braz. Chem. Soc., 16, 108-111 (2005). [ Links ]
2. Aubry, C.D., G. Chottard and N. Platzer, "Reinvestiga-tion of epoxidation using tungsten-based precur-sors and hydrogen-peroxide in a biphase medium," Inorg. Chem., 30, 4409-4415(1991). [ Links ]
3. Beare-Rogers, J., A. Dieffenbacher and J.V. Holm, "Lexicon of Lipid Nutrition (IUPAC Technical Report)", Pure Appl. Chem., 73, 685-744 (2001). [ Links ]
4. Bi, Y.L., M.J. Zhou and K.J. Zhen, "Oxidation of long Chain Primary Alcohols to Acids over the Quater-nary Ammonium Peroxotungstofosfato Catalyst System," React. Kinet. Catal. Lett., 72, 73-82 (2001). [ Links ]
5. Bortolini, O., V. Conte, F. Di Furia and G. Modena, "Metal Catalysis in Oxidation by Peroxides. Part 25. Molybdenum-and tungsten- catalyzed Oxida-tions of alcohols by diluted hydrogen Peroxide under Phase-transfer Conditions," J. Org. Chem., 51, 2661-2663 (1986). [ Links ]
6. Coupland, K. and P.J. Smith, European Patent EP0327379 (1989). [ Links ]
7. Guajardo, N., Estudio de la oxidación de docosanol a ácido docosanoico, M.Sc. Chemical Engineering Thesis, Pontificia Universidad Católica de Valparaíso (2007). [ Links ]
8. Hong, Z., Y.L. Bi, C. Tiexin, Z. Guangdong and Z. Kaiji, "Selective Oxidation of Octadecan-1-ol to Octa-decanoic Acid Over CO3O4/SiO2 Catalysts," React. Kinet. Cat. Lett, 81, 13-20 (2004). [ Links ]
9. Kan, Q.B., Y.L. Bi, Z. Ying, T. Wu and K. Zhen," Catalytic Oxidation of a- eicosanol into eicosanoic acid in the presence of Ti-MCM-41 or active component supported Ti-MCM-41 Catalysts," Microporous and Mesoporous Materials, 44-45, 609-617 (2001). [ Links ]
10. Lee, J., http: //www.hbci.com/~wenonah/new/pnutskin. htm, Access: 30 July 08 (2008). [ Links ]
11. Markovits, A., N. Guajardo, A. Olivares and G. Cea, "Proceso Para la Producción de Acido Lignocérico," Chilean invention patent, 1167-2009 (2009). [ Links ]
12. Markley, K.S., Fatty acids, Second edition, Inter-science Publishers Inc., New York (1960). [ Links ]
13. Plechkova, N. and K.R. Seddon, "Applications of ionic liquids in the chemical industry," Chemical Society Reviews, 37, 123-150 (2008). [ Links ]
14. Venturello, C. and M. Gambaro, "Selective Oxidation of alcohols and aldehydes with hydrogen Peroxide Catalyzed by Methyltrioctylammonium tetrakis (oxodiperoxotungsto)phosphate(3-) under two- Phase conditions," J. Org. Chem., 56, 5924-5931 (1991). [ Links ]
15. Venturello, C. and R. D'Aloisio, "Quaternary Ammonium Tetrakis (diperoxotungsto)phosphates(3-) as a New Class of Catalysts for Efficient Alkene Epoxidation with Hydrogen Peroxide," J. Org. Chem., 53, 1553-1557 (1988). [ Links ]
Received: March 10, 2011.
Accepted: March 12, 2012
Recommended by subject editor: Pedro de Alcântara Pessôa














