Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Latin American applied research
versión impresa ISSN 0327-0793versión On-line ISSN 1851-8796
Lat. Am. appl. res. vol.43 no.1 Bahía Blanca ene. 2013
Influence of reaction time on the properties of Zn2SnO4 nanoparticles obtained by hydrothermal method
E. L. Foletto†, D. S. Paz, J. M. Simões, S. Battiston, G. C. Collazzo, M. A. Mazutti, D. Bertuol and S. L. Jahn
Department of Chemical Engineering, Federal University of Santa Maria, Santa Maria-RS, 97105-900, Brazil. † E-mail: efoletto@gmail.com
Abstract— This work investigates the influence of reaction time (ranging from 12 to 96 h) on the characteristics of zinc stannate (Zn2SnO4) nanoparticles obtained by the hydrothermal method at 200°C. The nanoparticles were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), surface area measurements (BET) and infrared spectroscopy (IR). The results showed that the reaction time influenced the properties of Zn2SnO4 nanoparticles. The product presented a pure crystalline phase with mean particle size about 20 nm and BET surface area ranging from 34 to 43 m2.g-1.
Keywords— Zn2SnO4; Synthesis; Hydrothermal Process.
I. INTRODUCTION
Semiconductors composed by binary oxides of ZnO, SnO2, In2O3 and CdO are known by their excellent optical and electrics properties, being widely used in a variety of applications including smart windows, photovoltaic slim films and, optical and electronic nanodevices (Nomura et al., 2003; Liang et al., 2001). In the last years, the application as semiconductors of ternary nanostructured oxides was verified in several studies concerning the synthesis of these oxides. Among these materials the ternary zinc stannate oxide (Zn2SnO4) is known by its diamagnetic and semiconductor properties (Coffen, 1953).
The Zn2SnO4 is a spinelic material used in gas sensors (Yu and Choi, 2002), as an anode for Li-ion battery (Belliard et al., 2000) and as catalyst in the decomposition reaction of benzene in aqueous solutions (Wang et al., 2002). It is usually prepared by the solid-state reaction between ZnO and SnO2 at high temperatures (higher than 1000 °C) (Hashemi et al., 1990). However, the high temperature involved in the synthesis of Zn2SnO4 contributes to the loss of ZnO by evaporation and the formation of SnO2 that difficult the obtainment of a pure crystalline phase (Stambolova et al., 2005).
Some strategies to overcome these drawbacks were proposed in the literature. To avoid the partial evaporation of ZnO, different thermal treatments consisting of several steps and intermediary homogenization of product (Gupta and Mathur, 1968) were used. The formation of a pure phase of the spinel has been obtained after the mixture of ZnO and SnO2 at 1280°C (Hashemi et al., 1990). Mechanical activation by milling of ZnO and SnO2 ,previous to the thermal treatment at 1200 oC from 10 to 160 min, was also reported (Nikolic et al., 2001). Co-precipitation of the hydroxides Zn and Sn with NaOH from an aqueous solution containing ZnSO4 and SnCl4 at molar ratio of 2:1 was used. The precipitated was converted to Zn2SnO4 at 500-900 oC (Cun et al., 2002). Zn2SnO4 also has been formed after calcination of hydroxide precursor at temperature of 650-750°C. The hydroxide was obtained by the co-precipitation of Zn(NO3)2 and SnCl4 and using Na2CO3 as mineralizing agent. The product presented average particle size in the order of 20 nm after calcination at 650 oC for 24 h (Stambolova et al., 2005). Nanotubes of Zn2SnO4 were produced by thermal evaporation, at 1000 oC and 2 h, obtaining particles with diameter in the range of 50-100 nm (Wang et al., 2004a). Nanobelts and nanorings of Zn2SnO4 were also obtained by the same method of synthesis and under the same experimental conditions (Wang et al., 2004b). Nanoplates of Zn2SnO4 with a thickness of 50 nm were obtained by hydrothermal method (200 oC at 20 h of reaction time) employing hexadecil-trimethyl ammonium as surfactant (Ji et al., 2010). Nanowires of 50-100 nm of diameter were obtained by vapor chemical deposition method heating a mixture of metal Zn and Sn powders at 800-900 oC (Hu et al., 2009). Microtubes formed by ternary oxide were synthesized by the hydrothermal method (220 oC for 48 h), resulting in microtubes with diameter of 0.8 to 1.2 μm, which are composed of numerous nanoparticles with diameter of 10-20 nm and surface area of 41.2 m2.g-1 (Ai et al., 2010). The spinelic oxide also was synthesized using supercritical water in a bath reactor (400°C and 30 MPa) forming particles of 0.5-1.0 μm (Lee et al., 2010). The use of Na2CO3 as mineralizing agent under hydrothermal conditions in the range of 120-230 oC for up to 30 h resulted in the formation of Zn2SnO4 particles with diameters near 20-50 nm (Annamalai et al., 2010). Other studies reported the influence of different amines (ethylamine, n-butylamine, n-hexylamine and n-octylamine) as mineralizing agents on properties of resulting Zn2SnO4 particles at 180 oC for 20 h (Fu et al., 2009). Zn2SnO4 was synthesized via hydrothermal method at 220 °C for 72 h from zinc acetate and tin tetrachloride in various mediums containing different NaOH concentrations (0 to 6 mol.L-1). The optimum concentration for Zn2SnO4 crystallization was around 2.67 mol.L-1 (Fang et al., 2001).
Although there are several studies reporting different alternatives to synthesize the Zn2SnO4 there are no studies concerning the evaluation of the reaction time on the surface area of the nanoparticles obtained by the hydrothermal method.
In this sense, the main objective of this work was to investigate the influence of the reaction time on the characteristics of zinc stannate (Zn2SnO4) nanoparticles obtained by the hydrothermal method, focusing mainly on the effect in the surface area. The nanoparticles were characterized by X-ray diffraction, scanning electron microscopy, BET surface area measurements and infrared spectroscopy.
II. METHODS
A. Materials
The reagents used in the synthesis of zinc stannate (Zn2SnO4) nanoparticles were zinc acetate (Zn(CH3COO)2.2H2O), tin tetracloride (SnCl4.5H2O) and sodium hydroxide, all of them were of analytical grade.
B. Synthesis of Zn2SnO4
Zn2SnO4 nanoparticles were synthesized by the hydrothermal method at 200°C at different reaction times (12, 24, 48, 72 and 96 h). The molar composition of the reaction mixture was SnO2:2.33ZnO:360H2O:8.3NaOH.
The amount of each reagent was calculated according to the following stoichiometric reaction:
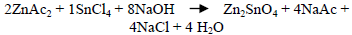 |
Solution of zinc acetate (12.85 g dissolved in 59 mL of water) was slowly added to the solution of tin tetrachloride (8.8 g dissolved in 50 mL of water) under magnetic stirring. Afterwards, NaOH 5.7 M was added until the solution reached pH 7.5. The formed gel was transferred to Teflon jars and placed in a stainless steel autoclave. The autoclave was placed in an oven previously heated to 200°C in order to carry out the hydrothermal treatment during 12, 24, 48, 72 and 96 h. Subsequently, the autoclave was removed from the oven and cooled in running water. The precipitate was washed with distilled water, centrifuged and dried in an oven at 100°C for 12 h. The solid sample obtained was finely ground using a mortar and pestle and stored in plastic containers.
C. Characterization of the Zn2SnO4 nanoparticles
Nanoparticles were characterized by X-ray diffraction, scanning electron microscopy, infrared spectroscopy and BET surface area measurements. X-ray diffraction (XRD) patterns were obtained using a Bruker D8 Advance Diffractometer. The X-ray source was Cu-Kα radiation, powered at 30 kV and 20 mA. Data were collected over the 2q range 20-80° with a step size of 0.05° and a count time of 35 s. The average nanoparticles size was determined using the Scherrer equation: D = K.λ/ (β.cosθ), where D is the average crystallite size, K is the Scherrer constant (0.90), λ is the wavelength of the X-ray radiation (0.15425 nm for Cu-Kα ), β is the peak width at half height and θ corresponds to the peak position (in the current study, 2θ = 34.29).
The morphology of Zn2SnO4 nanoparticles was determined by scanning electron microscopy using a Model Joel microscope. By means of infrared spectroscopy, infrared spectra of all samples pressed into KBr pellets were recorded by a Bruker tensor 27 FTIR spectrometer. IR spectra were measured in the range 1000-350 cm-1. The BET surface areas were obtained from nitrogen adsorption isotherms at 77 K, carried out on a Quantachrome Autosorb Automated Gas Sorption system, at relative pressure (P/Po) ranging from 0 to 0.99.
III. RESULTS AND DISCUSSION
X-ray diffraction was used to evaluate the crystalline structure of the synthesized nanoparticles. Figure 1 shows the XRD patterns of the prepared Zn2SnO4 samples obtained after the hydrothermal treatment at 200°C at different times, as well as data of the pure Zn2SnO4 (Card JCPDS no 74-2184). All diffraction peaks can be perfectly associated with a cubic spinelic structure of centered face, which is in agreement with the data of the reference (Card JCPDS no 74-2184). It is seen that the peaks position of the nanoparticles of Zn2SnO4 (synthesized and reference) are similar. This indicates the complete formation of a spinelic phase for all the samples synthesized in the different reaction times.

Fig. 1. XRD patterns of samples synthesized in different reaction times and card No. 74-2184 data of the reference for the Zn2SnO4.
Figure 1 evidenced that after 12 h of reaction is possible to obtain a spinelic phase in the crystalline form. However, with the increase of reaction time above 24 h, the peaks became slightly less intense, indicating a decreasing of crystallinity with a longer reaction time.
The hydrothermal route presented in this work presents as advantage the obtainment of the nano-particles of Zn2SnO4 with high cristallinity and purity at milder reaction conditions when compared to other processes, for example the conventional solid-state reac-tion method which requires high temperatures for the synthesis (above 1000oC) and some hydrothermal routes which need longer reaction times (more than 20 h).
Figure 2 presents the isotherm of nitrogen adsorp-tion/desorption of the nanoparticles of Zn2SnO4 synthe sized at 200°C after 12 h of reaction. Similar behaviorwas verified for all samples (data not shown). All adsorption and desorption curves showed a type II isotherm and are typical of microporous materials, according to the IUPAC classification.

Fig. 2. Nitrogen adsorption-desorption isotherms for Zn2SnO4 obtained at 12 h of reaction.
Table 1 presents the results concerning the surface area, average particle size and pore volume for nanoparticles obtained at all reaction times evaluated in this work. The mean particle size was calculated using the Scherrer equation. The particle size increased ranging from 19 to 22.5 nm, whereas the superficial area decreased from 43 to 34 m2.g-1 (a decrease of near 20 %) for reaction times ranging from 12 to 96 h. These results show that the reaction time has a reasonable influence on the surface area and a slight influence on the pore volume. The crystallite size increased as the contact time rose due to nanocrystal coalescence that takes place through the large amount of energy released. The increase of contact time favors the coalescence, being that the reaction time regulates the nanocrystal growth (Collazzo et al., 2011).
Table 1. BET Surface area, average particle size and total pore volume of the synthesized samples.

The average particle size and surface area obtained in this study are in agreement with those obtained in other studies. Stambolova et al. (2005) used NaCO3 as mineralizing agent and obtained particles of 20 nm after calcination at 650-750°C. Annamalai et al. (2010) obtained mean particle size in the range 20-50 nm using a hydrothermal process and NaCO3 as mineralizing agent. Ai et al. (2010) found a surface area of 41.2 m2.g-1 and diameter of 10-20 nm for the Zn2SnO4 microtubes synthesized at 220 oC for 48 h.
Figure 3 shows the micrographs of Zn2SnO4 samples treated hydrothermally at 200°C for reaction times of 12, 24, 48 and 72 h. SEM images indicates the formation of small aggregates of Zn2SnO4 nanocrystallites resulting in large particles with irregular shape. The surface morphology of samples obtained in longer reaction time is different than that of samples obtained in shorter reaction time. This can be more easily seen for the samples obtained at reaction time above of 24 h, which have larger particle size compared to the samples with reaction time of 12 and 24 h.
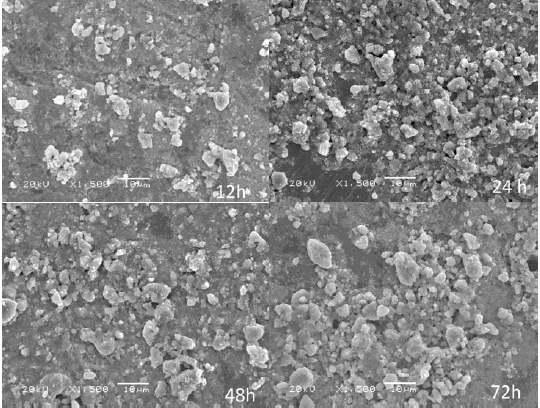
Fig. 3.Micrographs of samples obtained with different reaction times (12, 24, 48 and 72 h).
Figure 4 shows the infrared spectrum of Zn2SnO4 sample treated hydrothermally at 200°C only for 12 h of reaction in the region of 1000 to 350 cm-1. Similar behavior was verified for all samples (data not shown here).
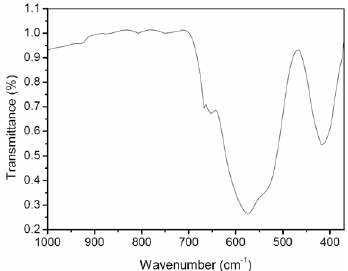
Fig. 4. Infrared spectrum of sample obtained at 12 h of reaction.
The absorption band at 572 cm-1 is assigned to the Zn2SnO4 spinel phase (Nikoli et al., 2007). The results obtained in the IR analysis confirmed the results obtained in the XRD analysis.
et al., 2007). The results obtained in the IR analysis confirmed the results obtained in the XRD analysis.
IV. CONCLUSIONS
The synthesis of zinc stannate oxide by hydrothermal process was investigated in this work. The results demonstrated that it is possible to obtain nanoparticles of Zn2SnO4 with mean size of 20 nm at 12 h of reaction. It was observed that the nanocrystallite size slightly increased with longer reaction times and as a consequence a decrease in the BET area occurred. The hydrothermal synthesis employed in this work present as advantage the obtainment of Zn2SnO4 nanocrystals in milder reaction conditions than those required in other processes. The results obtained in this work confirm that highly crystalline Zn2SnO4 nanocrystals can easily be synthesized at short reaction times.
REFERENCES
1. Ai, Z., S. Lee, Y. Huang, W. Ho and L. Zhang, "Photocatalytic removal of NO and HCHO over nanocrystalline Zn2SnO4 microcubes for indoor air purification," J. Hazard. Mat., 179, 141-150 (2010). [ Links ]
2. Annamalai, A., D. Carvalho, K.C. Wilson and M.-J. Lee, "Properties of hydrothermally synthesized Zn2SnO4 nanoparticles using Na2CO3 as a novel mineralizer," Mater. Characteriz., 61, 873-881 (2010). [ Links ]
3. Belliard, F., P.A. Connor and J.T.S. Irvine. "Novel Tin Oxide Based Anodes for Lithium Batteries," Solid State Ionics, 135, 163-167 (2000). [ Links ]
4. Collazzo, G.C., S.L. Jahn, N.L.V. Carreño and E.L. Foletto. "Temperature and reaction time effects on the structural properties of titanium dioxide nano-powders obtained via the hydrothermal method," J. Chem. Eng., 28, 265-272 (2011). [ Links ]
5. Coffen, W.W., "Ceramic and Dielectric Properties of the Stannates," J. Am. Ceram. Soc., 36, 207-214 (1953). [ Links ]
6. Cun, W., W. Xinming, Z. Jincai, M. Bixian, S. Guoying, P. Pingna and F. Jiamo, "Synthesis, characterization and photocatalytic property of nano-sized Zn2SnO4," J. Mat. Sci., 14, 2989- 2996 (2002). [ Links ]
7. Fang, J., A. Huang, P. Zhu, N. Xu, J. Xie, J. Chi, S. Feng, R. Xu and M. Wu, "Hydrothermal preparation and characterization of Zn2SnO4 particles," Mater. Res. Bull., 36, 1391-1397 (2001). [ Links ]
8. Fu, X., X. Wang, J. Long, Z. Ding, T. Yan, G. Zhang, Z. Zhang, H. Lin and X. Fu, "Hydrothermal synthesis, characterization, and photocatalytic properties of Zn2SnO4", Journal of Solid State Chemistry, 182, 517-524 (2009). [ Links ]
9. Gupta, M.P. and H.B. Mathur. "Mtissbauer spectra of oxidic spinels containing Sn4+ ion," J. Phys. Chem. Solids., 29, 1479-1481 (1968). [ Links ]
10. Hashemi, T., H.M. Al-Allak, J. Illingsworth and A.W. Brinkman, "Sintering behavior of zinc stannate," J. Mater. Sci. Letters., 9, 776-778 (1990). [ Links ]
11. Hu, Q.R., P. Jiang, H. Xu, Y. Zhang, S.L. Wang, X. Jia and W.H. Tang, "Synthesis and photoluminescence of Zn2SnO4 nanowires," J. Alloys Comp., 484, 25-27 (2009). [ Links ]
12. Ji, X., X. Huang, J. Liu, J. Jiang, X. Li, R. Ding, Y. Hu, F. Wu and Q. Li, "Hydrothermal synthesis of novel Zn2SnO4 octahedron microstructures assembled with hexagon nanoplates," J. Alloys Comp., 503, L21-L25 (2010). [ Links ]
13. Lee, J.-W. and C.-H. Lee, "Synthesis of Zn2SnO4 anode material by using supercritical water in a batch reactor," J. Superc. Fluids., 55, 252-258 (2010). [ Links ]
14. Liang, C.H., G.W. Meng, Y. Lei, F. Phillipp and L.D. Zhang, "Catalytic Growth of Semiconducting In2O3 Nanofibers," Adv. Mater., 13, 1330-1333 (2001). [ Links ]
15. Nikolic, N., T. Sreckovic and M.M. Ristic, "The influence of mechanical activation on zinc stannate spinel formation," J. Eur.Ceram. Soc., 21, 2071-2074 (2001). [ Links ]
16. Nikolic, M.V., T. Ivetic, K.M. Paraskevopoulos, K.T. Zorbas, V. Blagojevic and D. Vasiljevic-Radovic, "Far Infrared reflection spectroscopy of Zn2SnO4 ceramics obtained by sintering mechanically activated ZnO-SnO2 powder mixtures," J. Eur.Ceram.Soc., 27, 3727-3730 (2007). [ Links ]
17. Nomura, K., H. Ohta, K. Ueda, T. Kamiya, M. Hirano and H. Hosono, "Thin-film transistor fabricated in single-crystalline transparent oxide semiconductor," Science, 300, 1269-1272 (2003). [ Links ]
18. Stambolova, I., Y. Tsvetanova and P. Peshev, "Preparation of nanosized spinel stannate, Zn2SnO4, from a hydroxide precursor," J. Alloy. Compd., 391, L1-L4 (2005). [ Links ]
19. Wang, C., X. Wang and J. Fu, "Synthesis, characterization and photocatalytic property of nano-sized Zn2SnO4," J. Mater. Sci., 37, 2989-2996 (2002). [ Links ]
20. Wang, J.X., S.S. Xie, Y. Gao, X.Q. Yan, D.F. Liu, H.J. Yuan, Z.P. Zhou, L. Song, L.F. Liu, W.Y. Zhou and G. Wang, "Growth and characterization of axially periodic Zn2SnO4 (ZTO) nanostructures," J. Cryst. Grow., 267, 177-183(2004a). [ Links ]
21. Wang, J.X., S.S. Xie, H.J. Yuan, X.Q. Yan, D.F. Liu, Y. Gao, Z.P. Zhou, L. Song, L.F. Liu, X.W. Zhao, X.Y. Dou, W.Y. Zhou and G. Wang, "Synthesis, structure and photoluminescence of Zn2SnO4 single-crystal nanobelts and nanorings," Sol. State Commun., 131, 435-440 (2004b). [ Links ]
22. Yu, J.H. and G.M. Choi, "Selective CO gas detection of Zn2SnO4 gas sensor," J. Electroceram., 8, 249-255 (2002). [ Links ]
Received: July 17, 2011.
Accepted: February 29, 2012.
Recommended by Subject Editor María Luján Ferreira.














