Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Latin American applied research
versión impresa ISSN 0327-0793versión On-line ISSN 1851-8796
Lat. Am. appl. res. vol.43 no.1 Bahía Blanca ene. 2013
Thermal reactivation of a spent bleaching clay: kinetic and thermodynamic
G. C. Collazzo†, E. L. Foletto†, M.A. Mazutti†, D. Bertuol†, C.D.D Souza‡ and L.M. Porto‡
† Department of Chemical Engineering, Federal University of Santa Maria, Santa Maria-RS, 97105-900, Brazil.
‡ Department of Chemical Engineering and Food Engineering, Federal University of Santa Catarina, Florianópolis- SC, 88040-900, Brazil E-mail: gabicollazzo@hotmail.com
Abstract— Spent bleaching clay from the oil refining industry was subjected to heat regeneration in a box furnace with air. The heat reactivation was conducted without previous extraction of residual oil and with previous extraction of oil using organic solvent. The experimental temperature ranged from 723 at 873 K. A model for the kinetic of thermal reactivation of spent adsorbent has been proposed. The activation energy involved on the desorption phenomenon was determined.
Keywords— Spent Bleaching Clay; Thermal Reactivation; Kinetic.
I. INTRODUCTION
The removal of pigments and several other traces of constituents by adsorption is one of the most important stages in the oil refining process. Activated clays with strong inorganic acids have been used as adsorbents. However, the used clays, impregnated with oil, correspond to about 20-30 % of the total weight and are discarded in landing, causing problems of environmental pollution. The restrained unsaturated oils in the discarded clay oxidize quickly when in contact with air. This process causes strong odor and the material spontaneous combustion becomes possible (O'Brien, 1998). Thus, its disposal on the ground is a problem to be solved.
Methods to reactivate used bleaching clays using extraction with solvents have been suggested (Andersen, 1962; Foletto et al., 2002; Nursulihatimarsyila et al., 2010). Nag et al. (1997) have studied the effects of the reactivation of used clays on its structural properties. The removal of the residual oil was carried out by the use of organic solvents. Later, the samples have been submitted to acid and thermal treatment. Oil extraction of maize oil refinery using clay was studied by Al-Zahrani and Alhamed (1995) using different organic solvents. Excellent extraction conditions have been reported for each solvent. Other studies have evaluated the recycling of used clays by thermal treatment at high temperatures. The heating of the clay promotes complete removal of the residual oil and other present organic components adhered to clay particles. Solvent extraction followed by calcination at 500 °C is reported to be a method that produces more active clay than the original one (US Patent, 1999). Al-Zahrani and Daous (2000) have tested different organic solvents for removal of the residual oil, followed by calcination at different temperatures. They have found the optimal conditions of regeneration for commercial clay. A study involving thermochemical regeneration with ZnCl2 and heating under a rate of 10 °C.min-1 until 570°C, under nitrogen atmosphere for 1 h, showed that the regenerated clay presents good adsorbent properties (Tsai et al., 2003). Ma and Lin (2004) reported an efficiency of 94%, during the spent clay regeneration that is slightly lower than that was found by other researchers, Hou et al. (1999), that was 98%. As described previously, some methods for spent clay reactivation have received considerable attention. However, information about kinetic studies of thermal reactivation of used clays is scarce in literature.
In this context, the main objective of this work was to evaluate the reactivation kinetic of a spent bleaching clay. The activation energy involved on the desorption phenomenon was determined.
II. METHODS
Two samples of clays were provided by the Bunge Industry (Santa Catarina State, Brazil): a commercial virgin clay (bentonite) (Engelhard,U.S.A) and a spent clay, containing impregnated soybean oil. The chemical composition of the virgin clay (% by weight) is, determined by X-ray fluorescence (Philips PW 2400 Spectrophotometer): SiO2 (70.60); Al2O3 (9.44); Fe2O3 (2.89); CaO (3.14); Na2O (0.44); K2O (0.69); MnO (0.02); TiO2 (0.79); MgO (1.30); P2O5 (0.16); Fire Loss = 10.54%. Neutral soybean oil from the same industry was used to determine the efficiency of reactivation in this work.
The procedure of spent clay reactivation was done in two ways: a) the thermal reactivation was carried out without previous extraction of the residual oil; b) the thermal reactivation was carried out after the previous extraction of the oil using organic solvent. For a) approximately 3.0 g of spent clay sample was placed in an alumina crucible. After the temperature stabilization of the oven (Lavoisier, model 400D) (approximately 30 min, under air flow of 10-3 m3.s-1), some crucibles were placed in its interior and the reactivation time was measured. The crucibles have been withdraw in predetermined times for determination of the reactivation kinetic. The reactivation temperature ranged between 723 and 873 K. For b) a system of Soxhlet extraction was used to remove the oil impregnated in the adsorbent. Hexane (purity > 99.9 %) was used as solvent. The extraction was carried out during 5 h, after this time variation in the mass of recipient that contained the extracted residual oil was not observed. The solvent was recovered by distillation. The oil-free clay sample was later submitted to the calcination process. The procedure adopted for the thermal reactivation was identical to that described above. The assays of oil clarification have been carried out to estimate the kinetic parameters and to determine the model that better describes the process of clay reactivation. The clarification tests were carried out according to Foletto et al. (2001) and Foletto et al. (2006). Neutral (alkali refined) soybean oil (100 g) and commercial clay (1.0 g) or reactivated clay (equivalent mass of 1.0 g) have been transferred to a refining system to form the slurry. The slurry was heated to 100°C and kept at this temperature for 30 minutes, under vacuum of 120 mmHg absolute, at mechanical agitation of 1000 rpm and in nitrogen atmosphere to prevent oxidation of the oil. After this procedure, the slurry was filtered with filter paper under vacuum. Enough amount of filtered oil was collected for the determination of the color using a digital spectrophotometer (CELM model E-225D), in the wavelength of 420 nm (Foletto et al., 2003). An industrially refined oil sample was used as standard for the calibration of the device.
II. RESULTS AND DISCUSSION
The Fig. 1 shows the experimental results of the kinetic oil clarification, as well as the fitting results obtained in the process of the spent clay direct calcinations (without previously extraction of the residual oil). The points correspond to the experimental data, whereas the lines show the fitted kinetic model, represented in the Eq. 1.
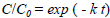 | (1) |
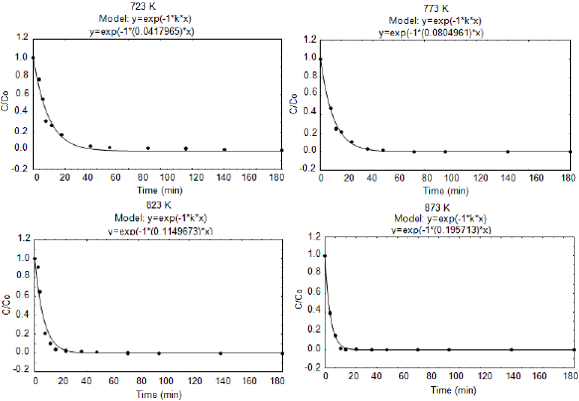
Fig.1. Fitting of the first order kinetic model for reactivated clay at different temperatures, without previous oil extraction.
where k is the first order kinetic constant and t the calcination time of the discarded clay sample. The concentration data have been treated in the dimensionless form (C/C0) and plotted in relation to the calcination time, being C0 the absorbance of the clarified oil by virgin clay and C the absorbance of the clarified oil by the reactivated clay. It is observed from Fig. 1 that the considered empirical model shows a good agreement with the experimental data.
During the calcinations, the active sites have been set free, and during the clarification test they were again occupied. This occupation was determined for the reduction in the concentration of the components that give color to the oil, that is proportional to the absorbance, based on the law of Beer-Lambert (Brimberg, 1982; Topallar, 1998). A fast decrease of C/C0 in the first points is observed, until it reduces to zero (C/C0 = 0), reaching the color of the oil used as standard (commercial refined soybean).
Figure 2 shows experimental results of the kinetic oil clarification with fitting curves for the calcination process of the spent clay with previously extraction of the residual oil. It is verified that the proposed model (Eq. 1) shows a good agreement with the experimental data. Table 1 shows the values of the kinetic constants in function of temperature for the two reactivation procedures investigated in this work. In the process without extraction a lower variation of the kinetic constant (k) is found with increasing of temperature comparing it to the process with extraction. Thus, a pretreatment with solvent makes the reactivation faster. In the experiments carried out with extraction it can be said that the clay is cleaner, favouring the process. Therefore, the process is faster, with kinetic constant approximately three times bigger; at 873K this value is of two times.

Fig. 2. Fitting of the first order kinetic model for reactivated clay at different temperatures, with previous oil extraction by hexane.
Table 1. Kinetic parameters determined by the first order kinetic model.

The activation energy involved on the desorption phenomenon was determined by the Arrhenius equation:
 | (2) |
where k is the kinetic constant, A is the factor of frequency or Arrhenius constant, Ea is the activation energy of thermal regeneration, R is the universal constant of gases (8.314 J.mol-1.K-1) and T is the absolute temperature. The slopes were calculated by a linear regression analysis of ln(k) versus 1/T (1/K), where is obtained a straight line with an intercept on the y-axis, giving ln (A), and a slope, giving - Ea/R (Fig. 3). The regression had a good linearity observing the coefficient r2. The value of Ea was 52.4 kJ.mol-1 for the process without previous oil extraction (with A = 4.67 s-1), and 43.8 kJ.mol-1 for the process with extraction (with A = 3.70 s-1). Thus, it is verified that the previous oil extraction with organic solvent improved slightly the reactivation process, since less activation energy is required. Only one work (Wang and Lin, 2000) that studied this term was found in the literature, the value found was 74.3 KJ.mol-1 for the reactivation of a spent clay containing residual oil of peanut.
Fig. 3. Activation energy of the clay regeneration process: (a) without residual oil extraction, (b) with previous extraction of the oil with hexane.
III. CONCLUSIONS
Observing the presented results it is possible to conclude that the reactivation efficiency of the spent clay increased with the increase of the calcination time. It was verified that the previous oil extraction with organic solvent slightly facilitates the reactivation process. Based in the experimental data, an empirical expression for the reactivation kinetic of spent bleaching clay was determined. The considered empirical model showed a good agreement with the experimental data. The activation energy involved on the desorption phenomenon was determined.
REFERENCES
1. Al-Zahrani, A.A. and Y.A.S. Alhamed, "Regeneration of spent bleaching clay and oil recovery by solvent extraction and acid treatment,"11th Int Conf on Solid Waste Techology and Management, Phiadelphia, USA, paper 7D (1995). [ Links ]
2. Al-Zahrani, A.A. and M.A. Daous, "Recycling of spent bleaching clay and oil recovery," Process Saf Environ, 78, 224-228 (2000). [ Links ]
3. Andersen, A.J.C., Refining of oils and fats for edible purposes, Pergamon Press, 2 ed., London (1962). [ Links ]
4. Brimberg, U.I., "Kinetics of bleaching of vegetable oils," J. Am. Oil Chem. Soc., 59, 74-78 (1982). [ Links ]
5. Foletto, E.L., C. Volzone, A.F. Morgado and L.M. Porto, "Obtenção e caracterização de materiais argilosos quimicamente ativados para utilização no descoramento de óleo vegetal," Mater. Res., 4, 211-215 (2001). [ Links ]
6. Foletto, E.L., C.C.A. Alves, L.R. Sganzerla and L.M. Porto, "Regeneration and utilization of spent bleaching clay," Lat. Am. Appl. Res., 32, 205-208 (2002). [ Links ]
7. Foletto, E.L., C. Volzone and L.M. Porto, "Performance of an Argentinian Acid-Activated Bentonite in the Bleaching of Soybean Oil," Braz. J. Chem. Eng, 20, 139-145 (2003). [ Links ]
8. Foletto, E.L., C. Volzone and L.M. Porto, "Clarification of cottonseed oil: how structural properties of treated bentonites by acid affect bleaching efficiency", Lat. Am. Appl. Res, 36, 37-40 (2006). [ Links ]
9. Hou, S.C., C.I. Lin and J.S. Hsu, "Heat regeneration of spent bleaching clay," J. Chin. Inst. Chem. Eng., 30, 501-503 (1999). [ Links ]
10. Ma, M.-H. and C.-I. Lin, "Adsorption kinetics of β-carotene from soy oil using regeneration clay," Sep Purif. Technol., 39, 201-209 (2004). [ Links ]
11. Nag, K.F., N.K. Nair, K.Y. Liew and A.M. Noor, "Surface and pore structure of deoiled acid- and heat-treated spent bleaching clays," J. Am. Oil Chem. Soc., 74, 963-970 (1997). [ Links ]
12. Nursulihatimarsyila, A.W., K.Y. Cheah and W.L. Chuah, "Deoiling and regeneration efficiencies of spent bleaching clay," Amer. J. Appl. Sci., 73, 434-437 (2010). [ Links ]
13. O'brien, R.D. Fats and oils, Technomic Publishing Company Inc., Lancaster, Pennsylvania (1998). [ Links ]
14. Topallar, H. "Bleaching kinetics of sunflowerseed oil", J. Am. Oil Chem. Soc., 75, 531-533 (1998). [ Links ]
15. Tsai, W.T., H.P. Chen, W.Y. Hsien, C.W. Lai and M.S. Lee, "Thermochemical regeneration of bleaching earth waste with zinc chloride," Resour Conserv Recy, 39, 65-77 (2003). [ Links ]
16. US Patent, Process for regenerating spent clay, Patent 5,942,457, August 24th (1999). [ Links ]
17. Wang, L.-H. and C. Lin, "Kinetics of heat regeneration spent bleaching clay", J Chem Eng. Jpn, 33, 522-525 (2000). [ Links ]
Received: November 24, 2011.
Accepted: May 17, 2012.
Recommended by Subject Editor María Luján Ferreira.














