Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Latin American applied research
versión impresa ISSN 0327-0793versión On-line ISSN 1851-8796
Lat. Am. appl. res. vol.43 no.2 Bahía Blanca abr. 2013
Influence of sodium silicate on floatability and charge of hematite and quartz with sodium oleate
D. R. Nascimento, R. D. Pereira and R. M. F. Lima
Mining Engineering Department, Universidade Federal de Ouro Preto, Campus Universitário do Morro do Cruzeiro, S/N., CEP. 35.400-000 - Ouro Preto - Minas Gerais, Brasil.
rosa@demin.ufop.br
Abstract— Due to its low cost compared with hydroxamate and sulphonate, sodium oleate is employed in direct flotation of low-grade or waste iron ore. However, depressants are being studied. This work shows the influence of the depressant sodium silicate on hematite and quartz flotation with sodium oleate as a collector. Microflotation tests were per-formed in Hallimond tube at pH values 7 and 9, and the zeta potentials of both minerals were determined in the absence and presence of sodium silicate. The sodium silicate was a more efficient depressant for quartz than for hematite, and a selective separation between the two minerals is possible for low reagent concentrations. The zeta potentials of both minerals were shifted to lower values due the specific adsorption of anionic species of sodium silicate onto their surfaces.
Keywords— Hematite; Quartz; Flotation; Sodium Silicate; Zeta Potential.
I. INTRODUCTION
Two possible flotation routes for iron ore are inverse flotation and direct flotation. For inverse flotation, the siliceous gangue (quartz) can be collected using cationic or anionic collectors with previous activation of quartz by hydroxyl complexes, such as CaOH+. In inverse flotation with cationic collector, the iron minerals are de-pressed by starch and starch products. The choice of the best flotation route and collector to concentrate a particular iron ore depends on the mineralogy (particle liberation size and the quantity of iron minerals, related to quartz or gangue minerals) and economics of the process flowsheet (Uwadiale, 1992). In the case of the world's largest reserves of iron ore, Brazilian itabirites, cationic flotation is frequently applied, using amine as the siliceous gangue collector and starch as a depressant of iron minerals at pH values ranging from approximately 10 to 10.5 (Araújo et al., 2003).
The first flotation route of iron ore to be tested and applied was direct flotation, using fatty acids, hydroxamate and sulphonate as iron minerals collectors (Houot, 1983). However, direct flotation that uses fatty acids as a collector is applied to low-grade or waste iron ore. Gangue depressants are being studied (Araújo et al., 2005).
In this work, the influence of sodium silicate on the flotation of hematite and quartz minerals using a sodium oleate collector was investigated.
II. METHODS
A. Sample and Reagents
Experiments were performed on hematite and quartz natural minerals from Quadrilátero Ferrífero, Brazil. First, the blocks of compact hematite sample were crushed in a jaw followed by a roll crusher and classified in a sieve of 1,700 µm. The fraction size below 1,700 µm was comminuted in a ball mill until all particles reached a size below 147 µm. The sand quartz was classified in a sieve of 147 µm, and only the particles above 147 µm were comminuted in a porcelain ball mill. The coarse fraction (-106 µm +43 µm) of both minerals was utilized in the microflotation tests. The finer fraction (-38 µm) was comminuted in an agate mortar to 90% minus 10 µm and utilized for the zeta potential measurements.
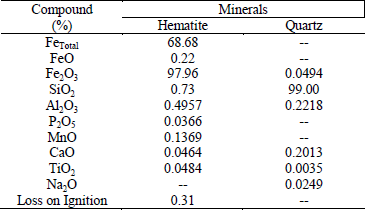
The chemical compositions of global samples and the fraction sizes of hematite from Quadrilátero Fer-rífero and the quartz sample studied by Lima et al. (2005) did not vary significantly. In this work, as the hematite sample was from Quadrilátero Ferrífero and the quartz sample was the same one studied by Lima et al. (2005), it was determined the chemical composition only of the global samples of hematite and quartz, comminuted below 38 µm (Table 1).
Table 1 - Chemical compositions and loss on ignition of natural mineral samples (global samples).
Reagents used in the microflotation tests and zeta potential measurements are presented in Table 2.
Table 2 - Reagents used in the microflotation tests and zeta potential measurements.

The stock solutions of sodium silicate and sodium chloride (1% w/v) were prepared by dissolution of 1 g of these reagents in 100 mL of distilled water. Preparation of the stock solution of sodium oleate (1% w/v) has been described by Andrade et al. (2012). The NaOH (5% w/v) and HCl solution (5% v/v), utilized in the pH control, were prepared by dissolving 5 g of (NaOH) and 5 mL of HCl in 100 mL of distilled water. All solution utilized in the microflotation tests and zeta potential measurements were prepared with distilled water.
B. Microflotation test
The microflotation tests were conducted in a Hallimond-modified tube at pH values of 7 and 9 using previously determined concentrations of sodium oleate that yield maximum (~100%) recovery for hematite (50 ppm) and quartz (70 ppm) (Lopes and Lima, 2009). The weight of each mineral sample was of 1 g, and the solution in the Hallimond tube was of 250 mL. First, the samples were conditioned with sodium silicate (modulus 1, manufactured by VETEC) for 6 min followed by 4 and 6 min of conditioning with sodium oleate for hematite and quartz, respectively. After conditioning, the mineral samples were floated for 1 min, using nitro-gen (flow rate of 60 mLmin-1).
C. Zeta potential determination
The zeta potentials of mineral samples were determined using the Malvern Zetasizer Nano Z - ZEN 2600. This equipment automatically determines the electrophoretic mobility of the particles and transforms it into a zeta (ζ) potential using Smoluchowiski's equation.
The suspension of each mineral (0.01% w/w) was prepared by the addition of 0.025 g of each mineral (d90 of 10 µm) into 250 mL of solution. Two different water suspensions of minerals were prepared in: 1 mM NaCl as the ionic medium; 1 mM NaCl and 0.1 mM sodium silicate. The suspension was settled for 20 min for hematite (density of 5,000 kg m-3) and 45 min for quartz mineral (density of 2,650 kg m-3) such that the supernatant particles had a size below 10 µm, according to Stokes' Law. The suspension was transferred to 25 mL beakers, and the pH was adjusted with hydrochloric acid (HCl) and sodium hydroxide (NaOH) under constant agitation in a magnetic shaker. The suspension pH was measured, and an aliquot from the suspension was slowly poured into the folded capillary cell to measure the zeta potential using the zeta meter. For each pH value, the zeta potential was determined as the average of the values obtained in two replicate measurements. The pH value of the suspension, under constant stirring, was measured after each zeta potential measurement was finished. Thus, the pH considered for each measurement was the value obtained at the end of each zeta potential test.
III. RESULTS
Hematite recoveries were almost constant up to 10 ppm of sodium silicate, and the depression (wettability) of the mineral was approximately 5 to 10% higher in pH 7 than in pH 9 (Fig. 1) up to this sodium silicate dosage. Nevertheless, the recovery of hematite in pH 9 reached a maximum with the tested dosage (50 ppm) (Lopes and Lima, 2009).
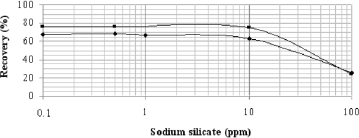
Fig.1. Recovery of hematite as a function of sodium silicate dosage; measurements were taken at pH 7 and 9 for a sodium oleate concentration of 50 ppm.
The isoelectric point (IEP) of hematite in a solution of sodium chloride was 7.4 (Fig. 2). This value is in agreement with the value determined in distilled water by Lopes and Lima (2009). When 0.1 mM sodium silicate was added to hematite suspension, the zeta potential of hematite in 1 mM NaCl for the pH range 6 to 11 shifted to lower values, indicating a specific adsorption of silicate anions with hematite surface. For an equilibrium time of seven days, using a complexation model, Jordan et al. (2007) proposed two types of sodium silicate complexes on the hematite surface as follows: ≡FeH3SiO4 and ≡FeH2SiO4-.

Fig. 2. Zeta potential of hematite as a function of pH.
As reported by Marinakis and Shergold (1985) for a dosage of 1 mM of sodium silicate at pH values below 9, silicic acid [H4SiO4] is the predominant specimen in solution. In this region, there is a smaller quantity of [SiO(OH)3-] that is predominant in a pH between 9.5 and 12.5. The specimen [SiO2(OH)22-] also appears at pH 6 and is predominant above pH 13. Thus, the higher depression of hematite mediated by sodium silicate at pH 7 compared with that at pH 9 could be attributed to the electrostatic attraction between the positive hematite surface at pH 7 and the anionic silicate specimens pre-sent in solution, once the zeta potential of the mineral in the presence of sodium silicate was negative in with a pH value near 7, as depicted in Fig. 2.
According to the model proposed by Jordan et al. (2007), the specimens [SiO(OH)3-] and [SiO2(OH)22-] present in solution form the complexes ≡FeH3SiO4 and ≡FeH2SiO4-, respectively on the hematite surface.
The quartz depression at pH 9 was constant for the concentrations of sodium silicate investigated and was higher compared to that at pH 7 (Fig. 3). However, the opposite finding was observed with hematite (Fig. 1). Despite the higher sodium oleate concentration in microflotation tests of quartz mineral (70 ppm) than hematite tests (50 ppm) (Figs. 1 and 3), the sodium silicate was more efficient to depress quartz (Fig. 3) than hematite (Fig. 1) for small dosages (up 10 ppm). Lopes and Lima (2009) verified the opposite behavior for sodium silicate dosage higher than 100 ppm.
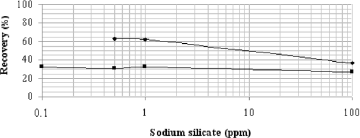
Fig. 3. Recovery of quartz as a function of sodium silicate dosage; measurements were taken at pH values of 7 and 9 for a sodium oleate concentration of 70 ppm.
The zeta potentials of quartz with 1 mM NaCl were negative for all pH values measured (Fig. 4). In the literature, the isoeletric point (IEP) of quartz is below pH 3 (Lopes and Lima, 2009, Iwasaki et al., 1960 and Kosmulski, 2011).
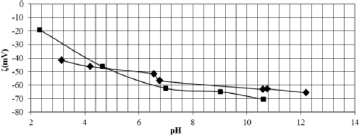
Fig. 4. Zeta potential of quartz as a function of pH.
The addition of 0.1 mM sodium silicate shifted the negative zeta potentials of quartz to lower values throughout the pH range that was investigated, except for the pH ranging from 2.4 to 4.8. This behavior could be related to silicic acid [H4SiO4] adsorbed onto the quartz surface, which is predominating at low pH values (Marinakis and Shergold, 1985). At pH values above 6, the predominant silicic specimens in solution are [SiO(OH)3-] and [SiO2(OH)22-], which specifically adsorbed onto the negative quartz surface and prevented sodium oleate adsorption, thus decreasing mineral recovery, as observed in microflotation tests (Fig. 3).
IV. CONCLUSIONS
The results show that sodium silicate is more efficient to depress quartz than hematite only at low concentrations (up 10 ppm). The lower recovery of hematite at pH 7 compared to that at pH 9 most likely is due to the electrostatic attraction of the silicates anions present in solution. The specimens [SiO(OH)3-] and [SiO2 (OH)22-] present in solution form the complexes ≡FeH3SiO4 and ≡FeH2SiO4-, respectively on the hematite surface. The zeta potentials of both minerals were shifted to lower values due the specific adsorption of anionic species of sodium silicate onto their surfaces.
ACKNOWLEDGMENTS
The financial support by the FAPEMIG, CNPq, Vale and CAPES is gratefully acknowledged.
REFERENCES
1. Andrade, E.M., B.L.C.M. Costa, G.A.G Alcântara and R.M.F. Lima, "Flotation of manganese minerals and quartz by sodium oleate/water glass," Latin American Applied Research, 42, 39-43 (2012). [ Links ]
2. Araújo, A.C., S.C. Amarante, C.C. Souza and R.R.R. Silva, "Ore mineralogy and its relevance for selection of concentration methods in processing of Brazilian iron ores," Mineral Processing and Ex-tractive Metalurgy (Trans. Inst. Min. Metall. C), 112, C44-C64 (2003). [ Links ]
3. Araújo, A.C., P.R. Viana and A.E. Peres, "Reagents in iron ore flotation," Minerals Engineering, 18, 219-224 (2005). [ Links ]
4. Houot, R., "Beneficiation of iron ore by flotation - re-view of industrial and potential applications," In-ternational Journal of Mineral Processing, 10, 182-204 (1983). [ Links ]
5. Iwasaki, I., S.R.B. Cooke and A.F. Colombo, Flotation Characteristics of Goethite, Washington United State of Interior, Berau of Mines, Report of investigation 5593 (1960).
6. Jordean, N., N. Marmier, C. Lomenech, E. Giffaut and J. Ehrhardt, "Sorption of silicates on goethite, hematite and magnetite: Experiments and model-ing," Journal of Colloid and Interface Science, 313, 224-229 (2007). [ Links ]
7. Kosmulski, V., "The pH-dependent surface charging and points of zero charge," Journal of Colloid and Interface Science, 353, 1-15 (2011). [ Links ]
8. Lima, R.M.F., P.R. Brandão and A.E. Peres, "The infrared spectra of amine collectors used in the flotation of iron ores," Minerals Engineering, 18, 267-273 (2005). [ Links ]
9. Lopes, G.M. and R.M.F. Lima, " Flotação direta de mi-nério de ferro com oleato de sódio," REM: R. Esc. Minas, 62, 323-329 (2009). [ Links ]
10. Marinakis, K.I. and H.L. Shergold, "Influence of Sodium Silicate Addition on the Adsorption of Oleic Acid by Fluorite, Calcite and Barite," International Journal of Mineral Processing, 14, 177-193 (1985). [ Links ]
11. Uwadiali, G.G.O.O., "Flotation of Iron Oxides and Quartz - A Review," Mineral Processing and Ex-tractive Metallurgy Review, 11, 129-161 (1992). [ Links ]
Received: February 24, 2012
Accepted: October 2, 2012
Recommended by Subject Editor: Mariano Martín














